Drug Property Introduction
>Boiling Point:
The temperature at which a liquid boils and turns to vapor.
>Caco-2 Permeability:
Caco-2 is a human colon epithelial cancer cell line. The permeability coefficients across monolayers of Caco-2, cultured on permeable supports, are commonly used to predict the absorption of orally administered drugs and other compounds.
>Log P:
The partition coefficient (P) is defined as a particular ratio of the concentrations of a solute between the two solvents, specifically for un-ionized solutes, and the logarithm of the ratio is thus Log P. When one of the solvents is water and the other is a non-polar solvent, then the log P value is a measure of lipophilicity or hydrophobicity. Octanol-water partition ratio is the most common way of expressing the lipophilicity of a compound, and it is defined as the ratio of the concentration of a solute in a water-saturated octanolic phase to its concentration in an octanol-saturated aqueous phase.
>Log S:
The logarithm value of solubility.
>Melting Points:
The melting point of a substance is the temperature at which it changes state from solid to liquid.
>pKa:
The pKa of a drug is the hydrogen ion concentration (pH) at which 50% of the drug exists in its ionized hydrophilic form (i.e., in equilibrium with its un-ionized lipophilic form). At physiologic pH, the lower the pKa the greater the lipophilicity.
>Water Solubility:
Water solubility is a measure of the amount of chemical substance that can dissolve in water at a specific temperature. The aqueous solubility of a compound significantly affects its absorption and distribution characteristics. Typically, a low solubility goes along with a bad absorption.
>Absorption:
Absorption is the transportation of a drug from the site of administration to the site of action. Absorption, which indicates how quickly and how much of a drug reaches the intended target, is a crucial factor affecting availability/bioavailability.
>AUC (Area under curve):
The area under the plot of plasma concentration of drug (not logarithm of the concentration) against time after drug administration. The AUC is of particular use in estimating bioavailability of drugs, and in estimating total clearance of drugs. The ratio of the AUC after oral administration of a drug formulation to that after the intravenous injection of the same dose to the same subject is used during drug development to assess a drug’s oral bioavailability.
>Bioavailability:
The percent of dose entering the systemic circulation after administration of a given dosage form. More explicitly, the ratio of the amount of drug “absorbed” from a test formulation to the amount “absorbed” after administration of a standard formulation. Frequently, the “standard formulation” used in assessing availability is the aqueous solution of the drug, given intravenously.
>C Max:
The maximum or “peak” concentration of a drug observed after its administration.
>Clearance:
The Clearance of a chemical is defined as the volume of body fluid from which the chemical is, apparently, completely removed by biotransformation and/or excretion, per unit time. In fact, the chemical is only partially removed from each unit volume of the total volume in which it is dissolved. Since the concentration of the chemical in its volume of distribution is most commonly sampled by analysis of blood or plasma, clearances are most commonly described as the “plasma clearance” or “blood clearance”.
>Css:
The concentration of a drug or chemical in a body fluid - usually plasma - at the time a steady state (the rates of drug administration and drug elimination are equal) has been achieved.
>Volume of Distribution:
The volume into which a drug appears to have been dissolved after administration to an organism. If one knew the mass (dose) of drug administered and the average concentration of the drug in the body, the apparent volume into which the drug had been dissolved could be determined from the relationship or definition: concentration = mass/volume. Since these idealized conditions are unobtainable in practice, the volume of distribution of a drug can only be approximated using experimental data.
>Eliminate Route:
Elimination Route of a drug is the process by which a drug is eliminated from an organism. The kidney is the main excretory organ, and others exist such as the liver, the skin, the lungs or glandular structures (e.g. the salivary glands and the lacrimal glands). These organs or structures use specific routs to expel a drug from the body.
>Half-life:
The period of time required for the concentration or amount of drug in the body to be reduced to exactly one-half of a given concentration or amount.
>Metabolic:
Drug metabolism is the metabolic breakdown of drugs by living organisms. The rate of metabolism affects the duration and intensity of a drug’s pharmacologic action.
>Protein Binding:
Protein binding refers to the degree to which medications attach to proteins. Protein binding can involve plasma proteins, extracellular tissue proteins, or intracellular tissue proteins. Plasma and tissue protein binding of drugs is a major factor that affects both pharmacokinetics and pharmacodynamics of the drug.
>T Max:
T Max is the time at which the C Max is observed.
>Toxicity LD50:
In toxicology, LD50 is a measure of the lethal dose. This value of a drug is the dose required to kill half the members of a tested population after a specified test duration.
>Toxicity LDLo:
The LDLo or Lowest Lethal Dose value is the lowest amount of a substance reported to have caused the death of animals or humans.
>Toxicity Lethal Dose:
The LDLo or Lowest Lethal Dose value is the lowest amount of a substance reported to have caused the death of animals or humans.
>Toxicity MRLs:
In toxicology, the MRL is an estimate of the amount of a chemical a person can eat, drink or breathe each day without a detectable risk to health.
>Toxicity TDLo:
The Lowest Published Toxic Dose (TDLo) is the lowest dosage per unit of bodyweight of a substance known to have produced signs of toxicity in a particular animal species.
>Tss:
Time to reach steady state.
Reference:
The largest dose of a medicine or drug consistent with safety in a certain population.
>Neonates, Infants, Children, Adolescents, Adults, Elderly, Geriatric.
DDPD Function Introduction

Fuzzy query is enabled. In the main page, drugs can be quickly searched either by its Identification or through approximate string matching.
Search result page
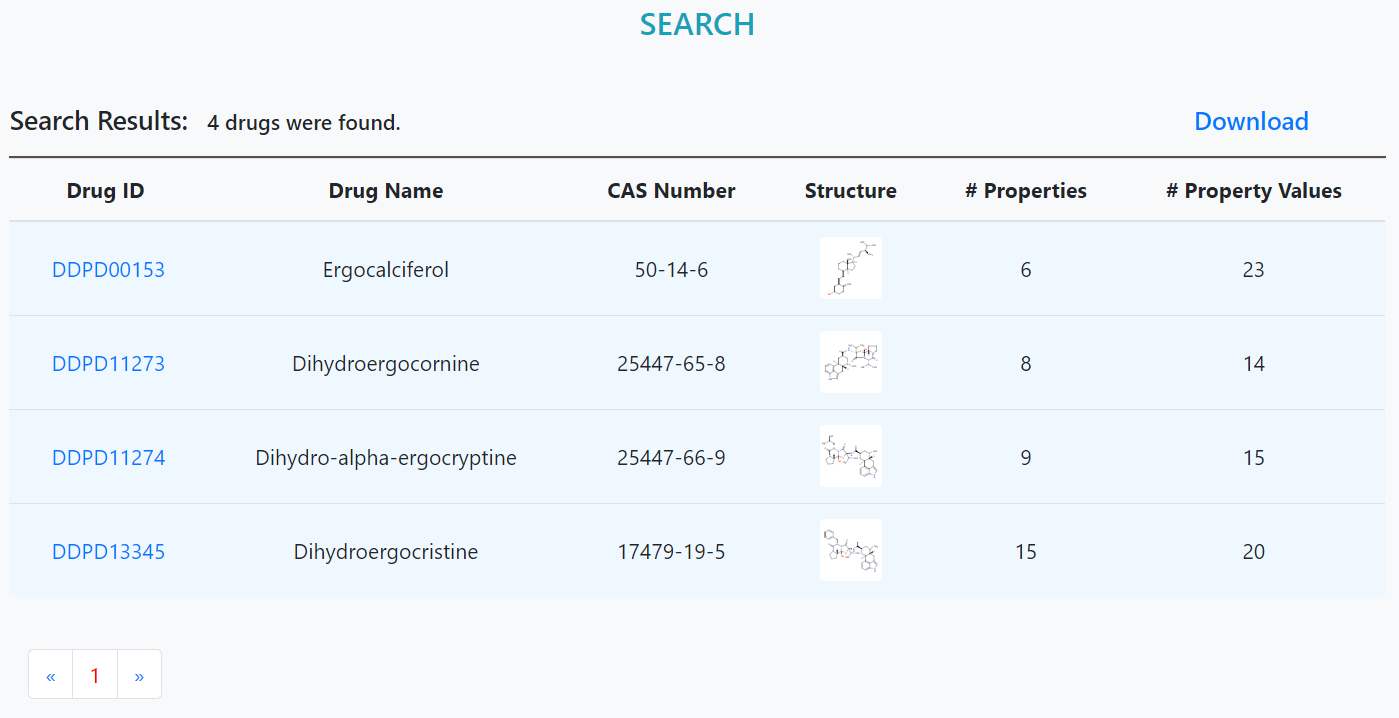
Drugs meeting the criteria are listed. Clicking on the drug ID will lead to the detailed information page.
Detailed information page
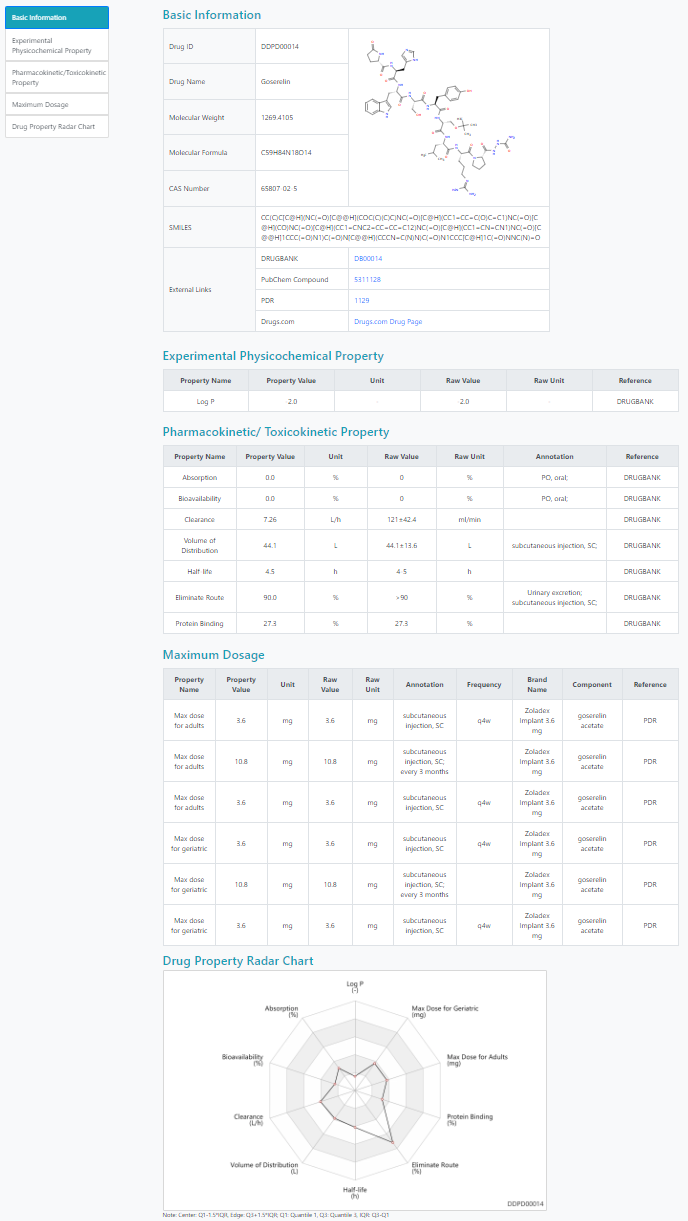
The detailed information page contains the available basic information, physicochemical properties, pharmacokinetic/toxicokinetic properties and maximum dosages of the drug.
A drug property radar chart displays property values over multiple drug properties. For each drug property, the center of the chart represents the 1.5*IQR (the interquartile range) below the first quartile of property values, and the edge 1.5*IQR above the third quartile.
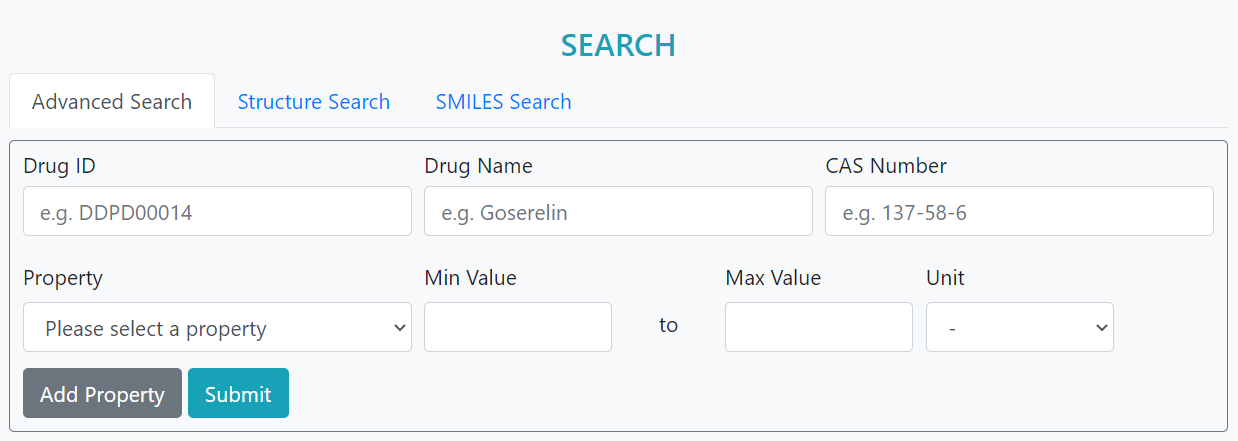
>Advanced Search
Advanced and more accurate search was developed based on the matchings of drug features including Drug ID, Drug Name, Boiling Point, Melting Point, ADMT properties and Max Dosage.
Step 1: Input ID and/or Name
Step 2: Add features, select units, and input values
Step 3: Submit
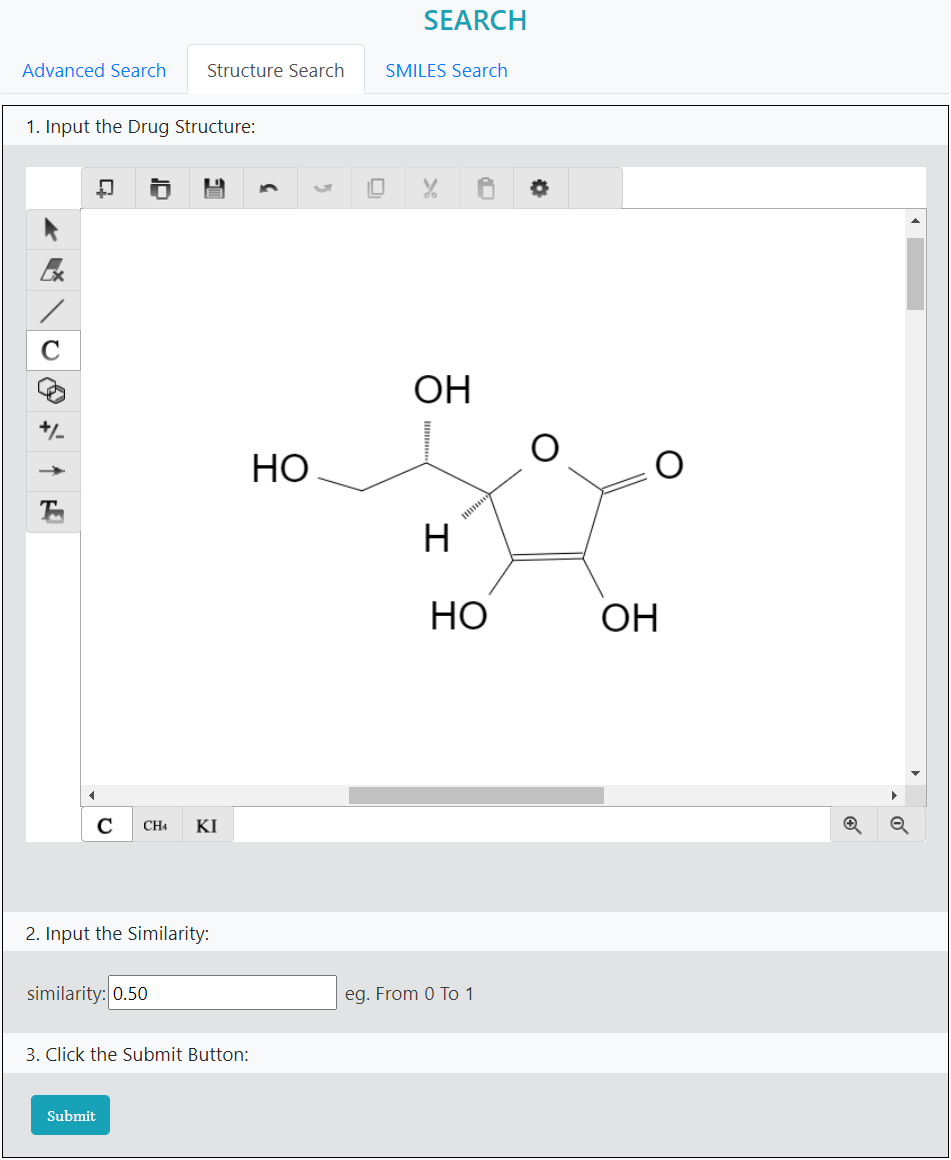
>Structure Search
Drug search based on chemical structure.
Step 1: Draw the structure manually using the integrated tool
Step 2: Input the similarity value
Step 3: Submit
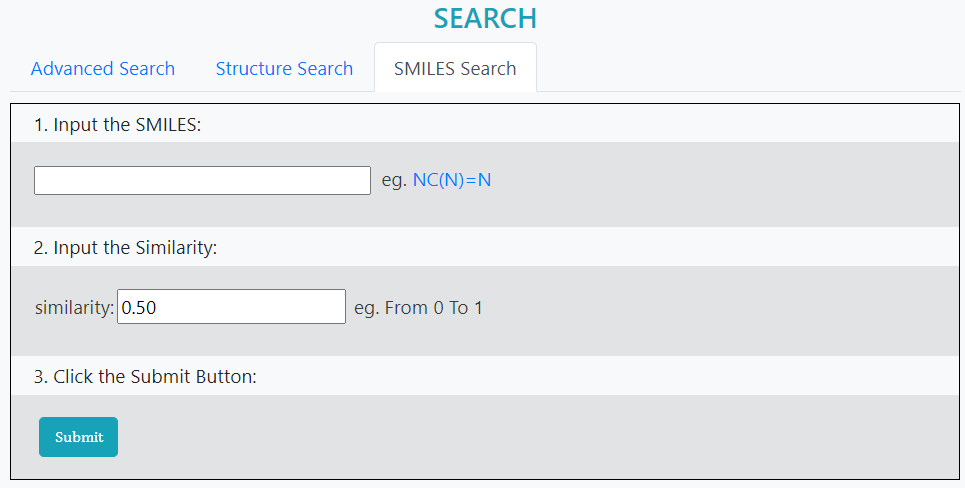
>SMILES Search
Drug search through SMILES string matching.
Step 1: Input SMILES
Step 2: Input similarity value
Step 3: Submit
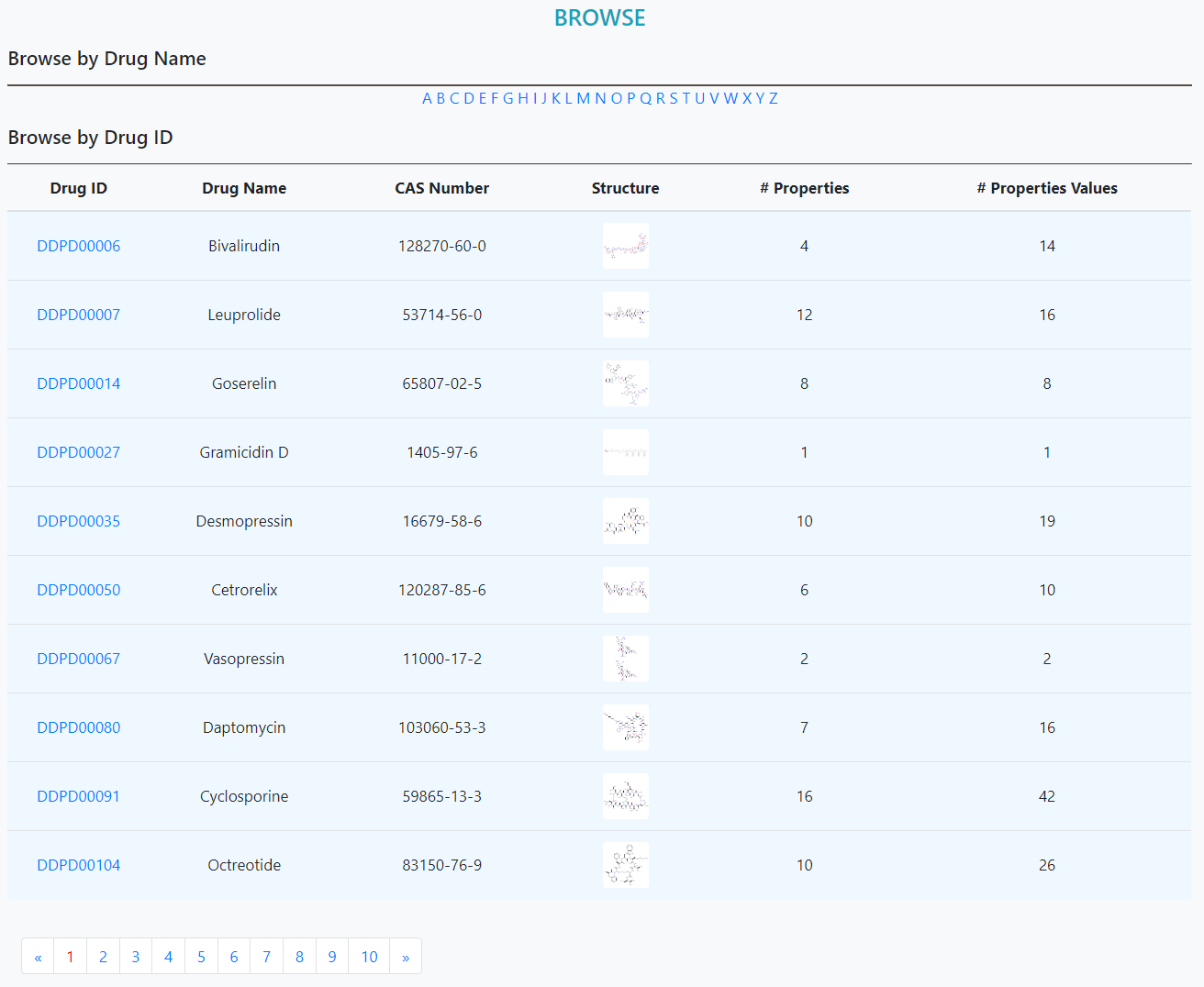
The list of drugs could be viewed on this page. Specifically, two options of browsing are provided, the drug list could be sorted by either drug ID numbers or the initials. The images of the chemical structures can be enlarged when the mouse is within the window area of the image.
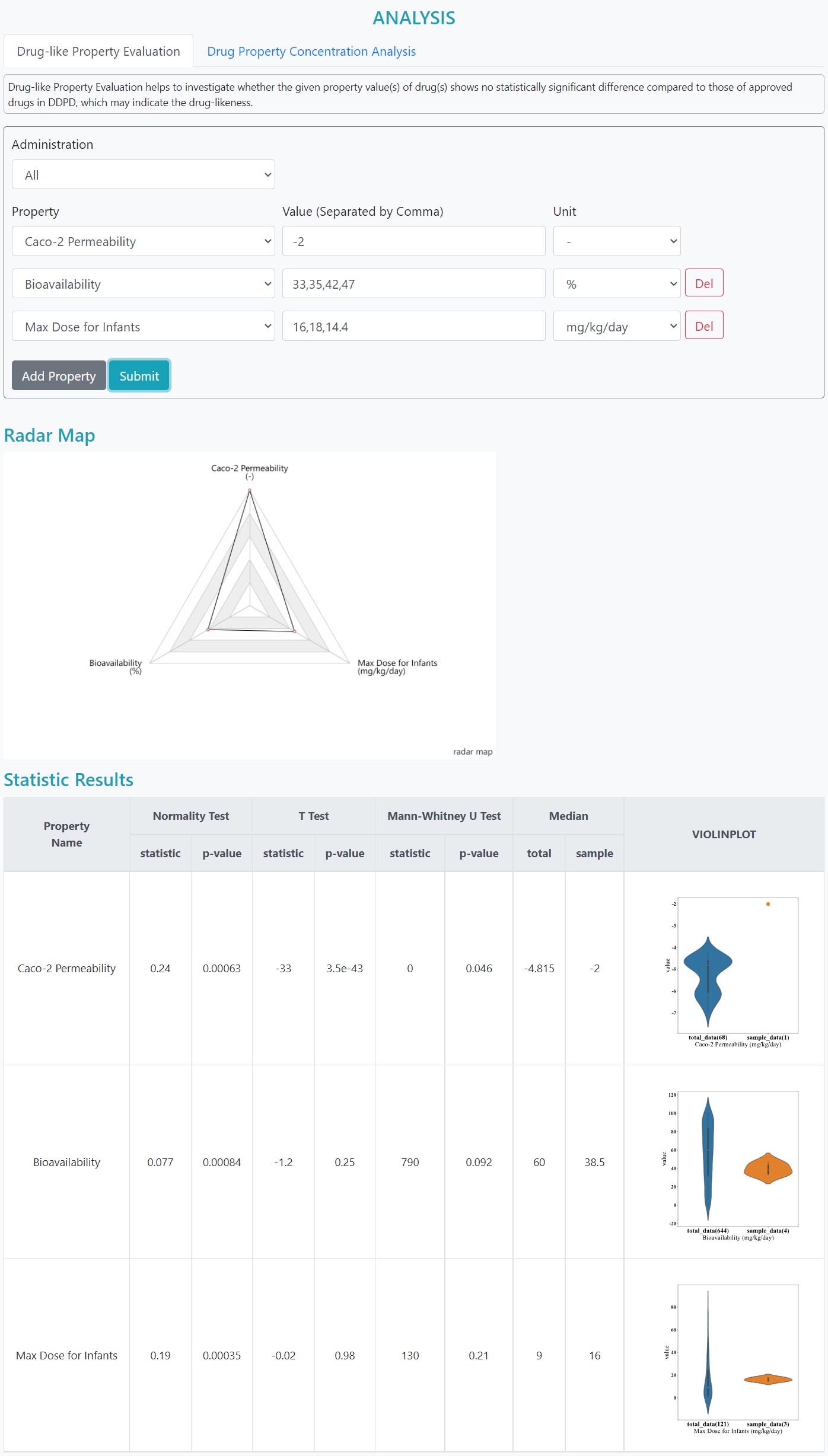
>Drug-like Property Evaluation
Drug-like Property Evaluation helps to investigate whether the given property value(s) of drug(s) shows no statistically significant difference compared to those of approved drugs in DDPD, which may indicate the drug-likeness.
Step 1: Select mode of administration, which includes P.O., I.V. or ALL
Step 2: Add features. Be remined that redundant feature entries are not accepted
Step 3: Input the values of the features, multiple items should be separated using commas, e.g., “3”, “14,18,52”
Step 4: Select the unit
Step 5: Submit
The results of the normality test, T test and Mann-U test are given. Violin plots are shown to visualize the distribution of the queried features of both the whole dataset and the input.
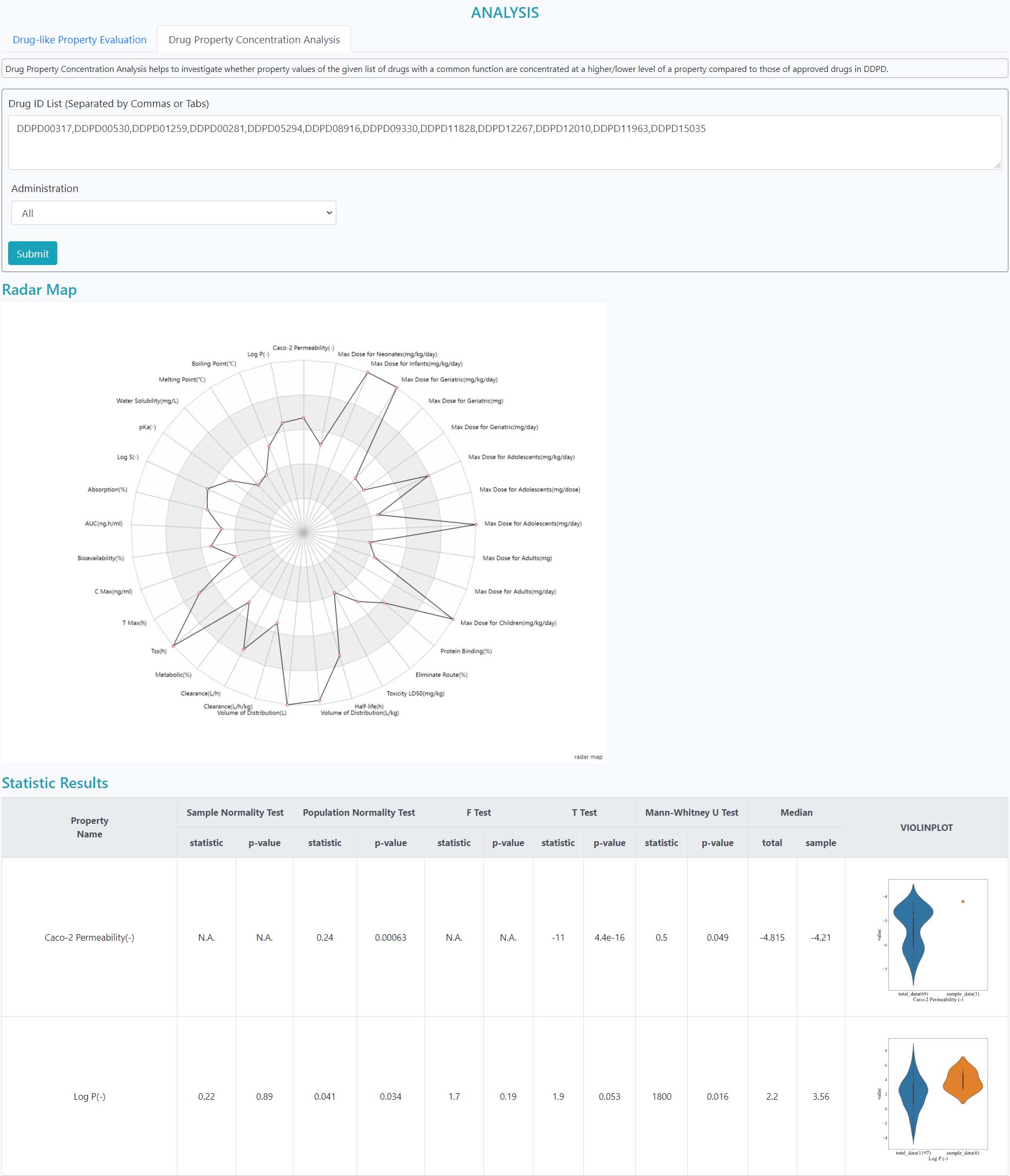
>Drug Property Concentration Analysis
Drug Property Concentration Analysis helps to investigate whether property values of the given list of drugs with a common function are concentrated at a higher/lower level of a property compared to those of approved drugs in DDPD.
Step 1: Input the drug list
Step 2: Choose the model of administration and submit
In the results, the normality tests of both the sample and the whole population are given. Other statistical test results are also included to indicate the difference between the distributions of the sample and the population. Violin plots are shown for visualizing the distributions
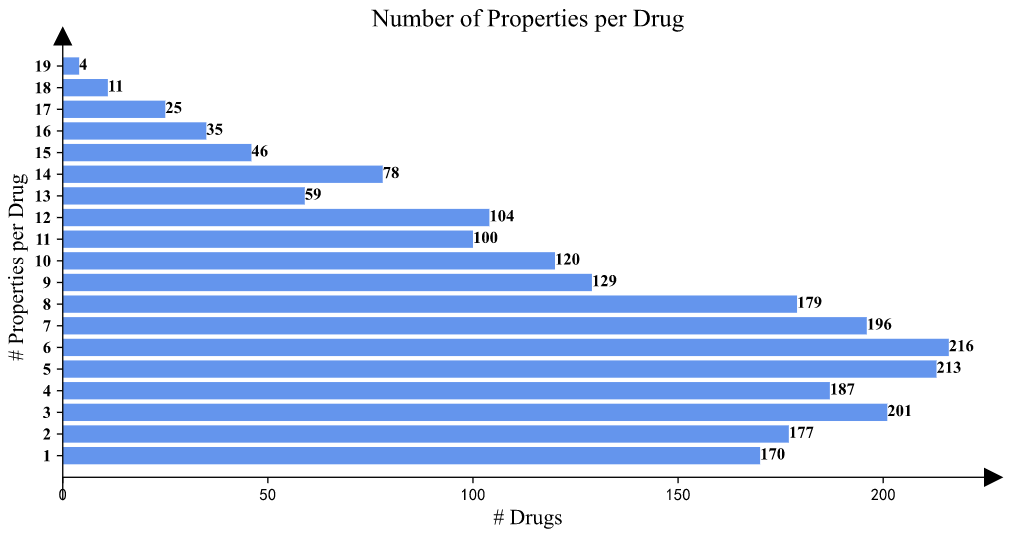
>Drug Property Statistics
In the "Drug Property Statistics" part, the statistics of the properties and the property values per drug are illustrated.
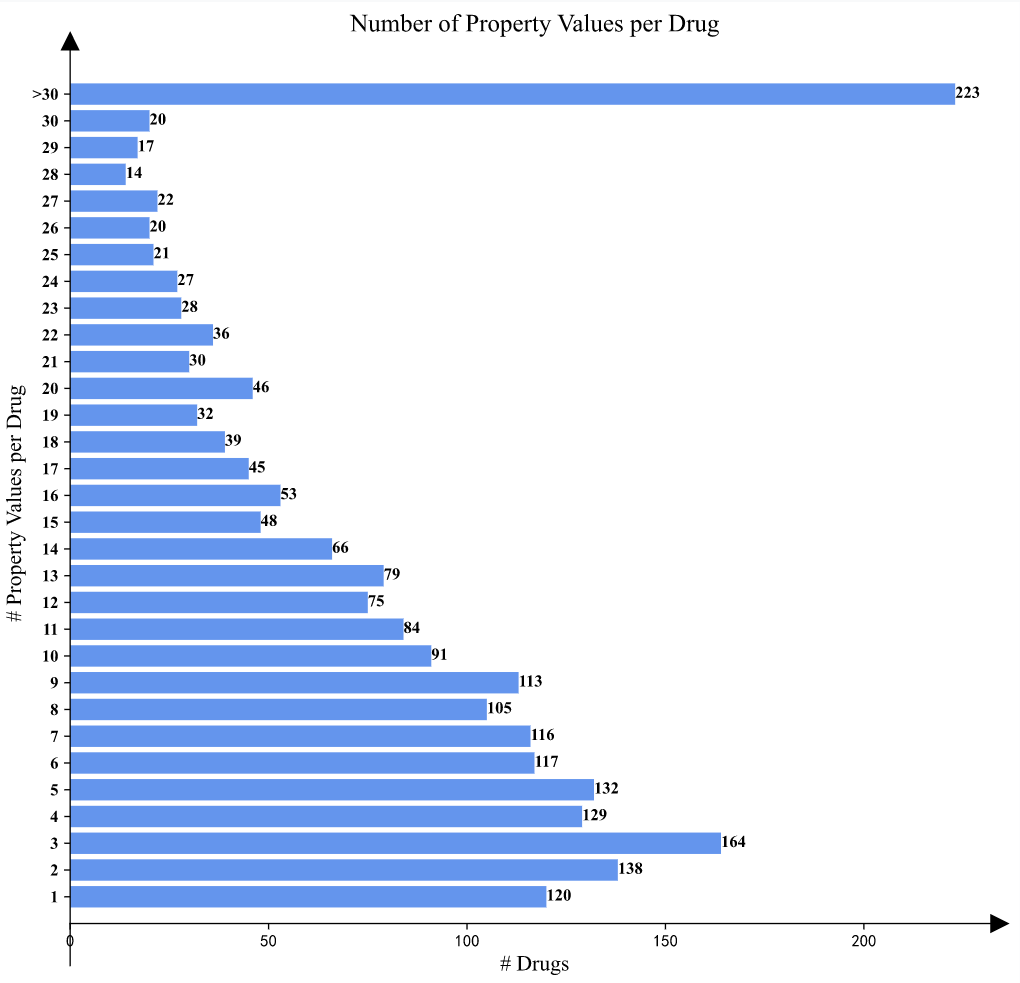
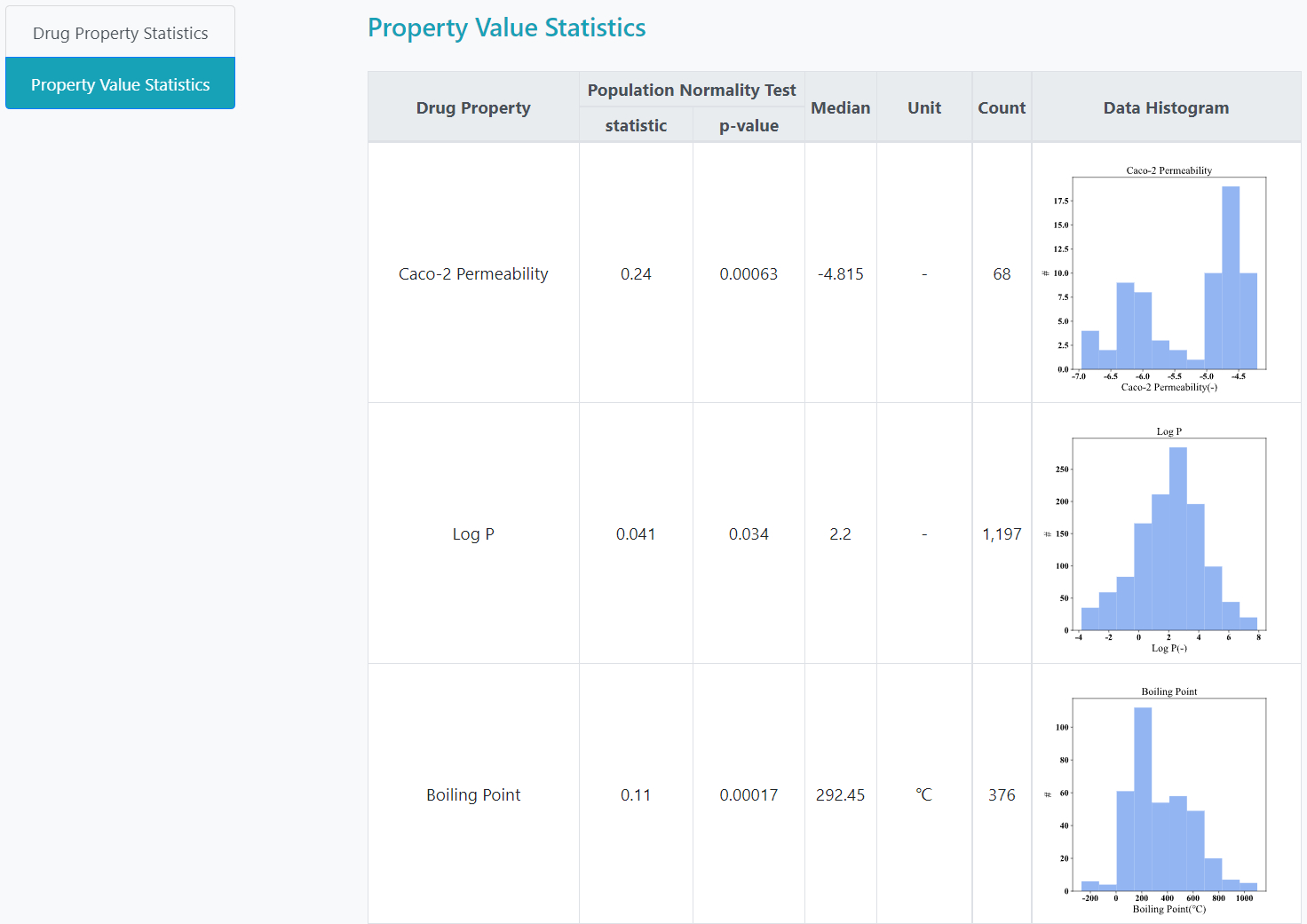
>Property Value Statistics
In the "Property Value Statistics" part, histograms of the features are shown to illustrate the distributions more directly. The normality test results are also included as well.
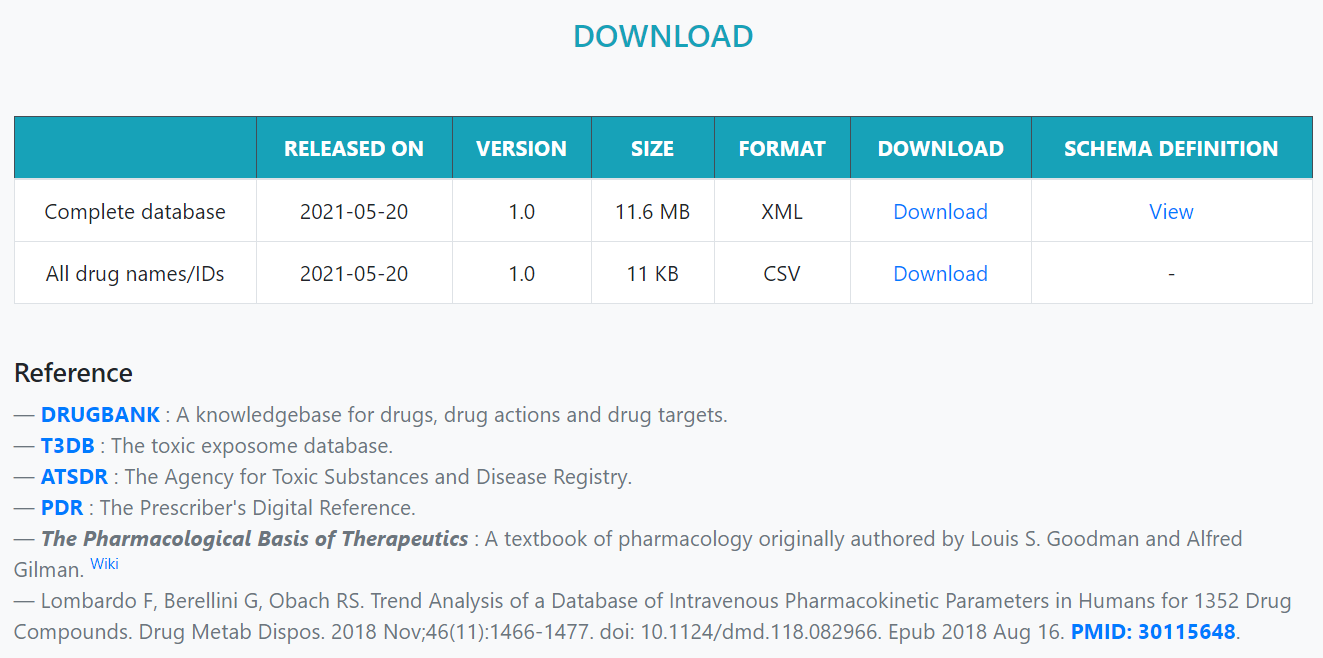
In the Download page, the entire data can be downloaded in XML format. The structure explanation file is also provided in the XSD format.
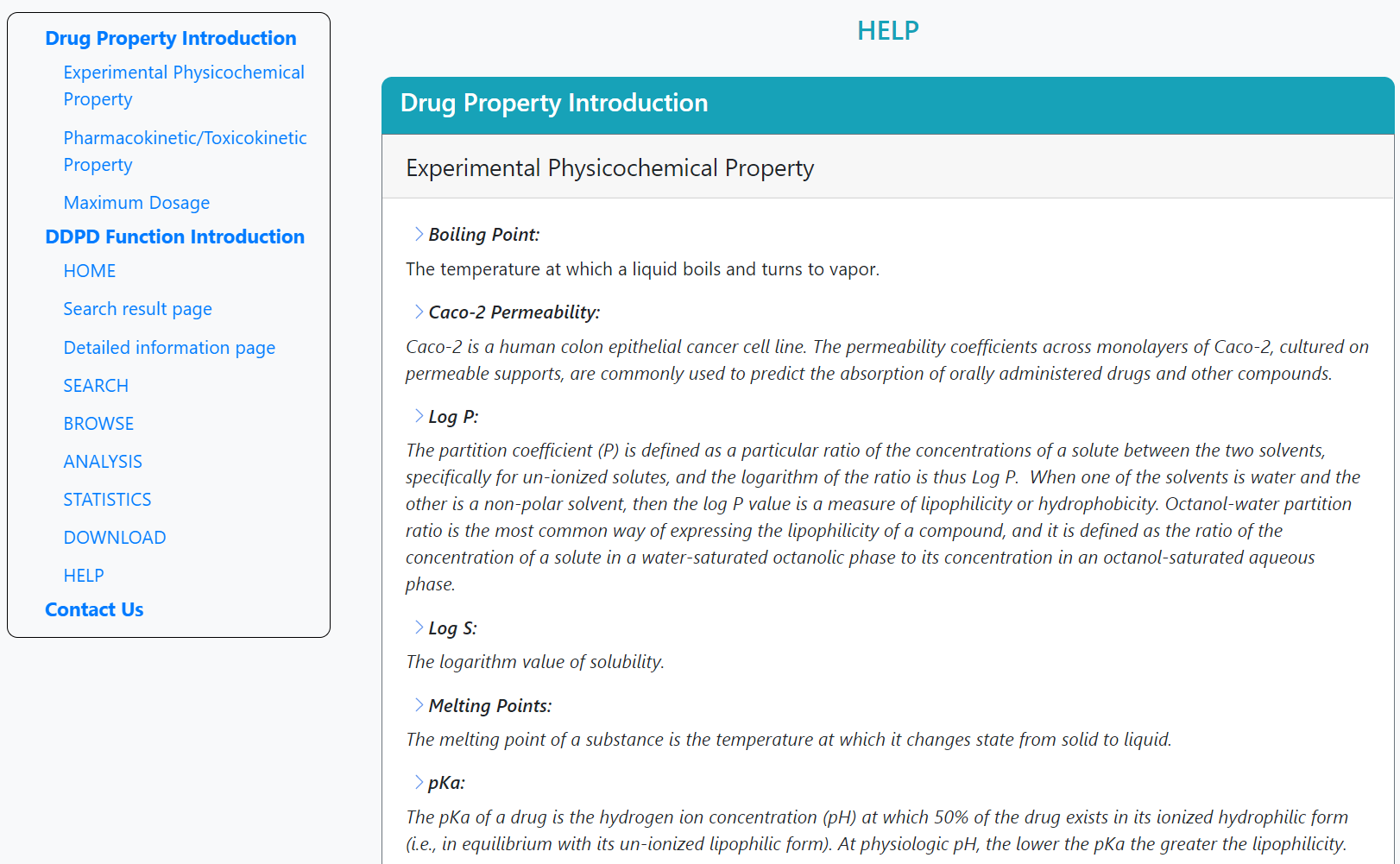
On the help page, the introduction of the features and the guidelines for the functions are stated. The contact details are attached at the bottom.
Contact Us
- Please feel free to contact Prof. Jianbo Pan with respect to any details pertaining to DDPD.
-
>Address :
Center for Novel Target and Therapeutic Intervention, Institute of Life Sciences, Chongqing Medical University,
No. 1 Yixueyuan Road, Yuzhong District, Chongqing, 400016, P. R. China.
-
>Email :
panjianbo@cqmu.edu.cn