Basic Information
| Drug ID | DDPD13928 |

|
| Drug Name | Semaglutide | |
| Molecular Weight | 4113.641 | |
| Molecular Formula | C187H291N45O59 | |
| CAS Number | 910463-68-2 | |
| SMILES | CC[C@H](C)[C@H](NC(=O)[C@H](CC1=CC=CC=C1)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCCNC(=O)COCCOCCNC(=O)COCCOCCNC(=O)CC[C@@H](NC(=O)CCCCCCCCCCCCCCCCC(O)=O)C(O)=O)NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CCC(N)=O)NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC1=CC=C(O)C=C1)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CC1=CC=CC=C1)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)C(C)(C)NC(=O)[C@@H](N)CC1=CNC=N1)[C@@H](C)O)[C@@H](C)O)C(C)C)C(=O)N[C@@H](C)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(O)=O | |
| External Links | ||
| DRUGBANK | DB13928 | |
| PDR | 24167 | |
Experimental Physicochemical Property
Not Available
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
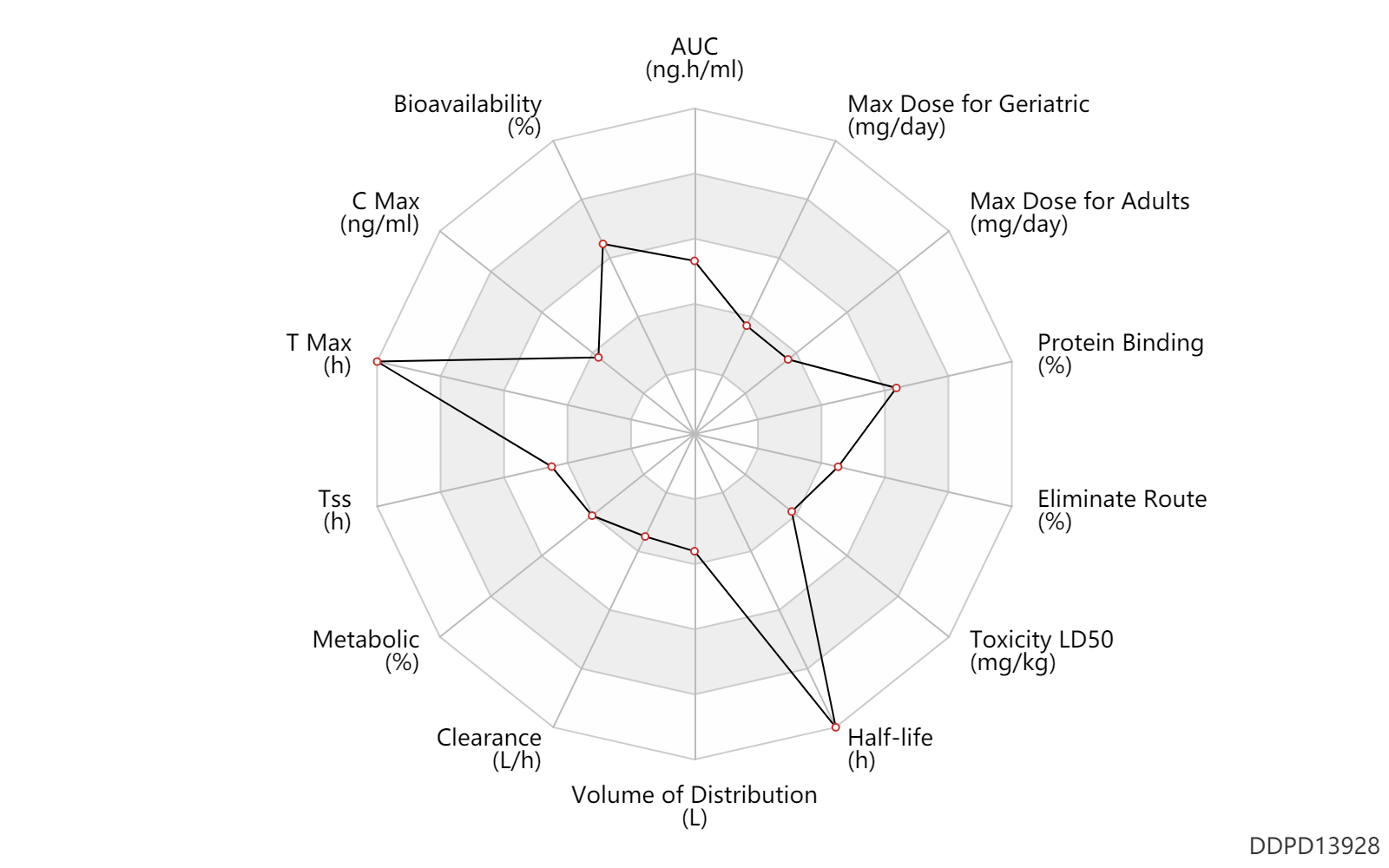
| AUC | 12848.5 | ng.h/ml | 3123.4 | nmol.h/L | DRUGBANK | |
| Bioavailability | 89.0 | % | 89 | % | DRUGBANK | |
| C Max | 44.8 | ng/ml | 10.9 | nmol/L | DRUGBANK | |
| Css | 94.6 | ng/ml | 16-30 | nmol/L | DRUGBANK | |
| T Max | 56.0 | h | 56 | h | DRUGBANK | |
| Tss | 108.0 | h | 4-5 | day | Tablet, PO, oral; | DRUGBANK |
| Metabolic | 7.7 | % | 7.7 | % | DRUGBANK | |
| Clearance | 0.0390 | L/h | 0.039 | L/h | DRUGBANK | Clearance | 0.0500 | L/h | 0.05 | L/h | type 2 diabetes; patients; | DRUGBANK |
| Volume of Distribution | 8.7 | L | 8-9.4 | L | DRUGBANK | |
| Half-life | 168.0 | h | 168 | h | plasma proteins; | DRUGBANK |
| Toxicity LD50 | 270.0 | mg/kg | 270.0 | mg/kg | PO, oral; Rattus, Rat; | DRUGBANK |
| Eliminate Route | 53.0 | % | 53 | % | Urinary excretion; | DRUGBANK | Eliminate Route | 18.6 | % | 18.6 | % | Faeces excretion; | DRUGBANK | Eliminate Route | 3.2 | % | 3.2 | % | lung excretion; | DRUGBANK |
| Protein Binding | 99.0 | % | >99 | % | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for adults | 0.142857142857143 | mg/day | 1 | mg/week | subcutaneous injection, SC | Ozempic | semaglutide | PDR |
| Max dose for adults | 14.0 | mg/day | 14 | mg/day | PO, oral | Ozempic | semaglutide | PDR |
| Max dose for geriatric | 0.142857142857143 | mg/day | 1 | mg/week | subcutaneous injection, SC | Ozempic | semaglutide | PDR |
| Max dose for geriatric | 14.0 | mg/day | 14 | mg/day | PO, oral | Ozempic | semaglutide | PDR |