Basic Information
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
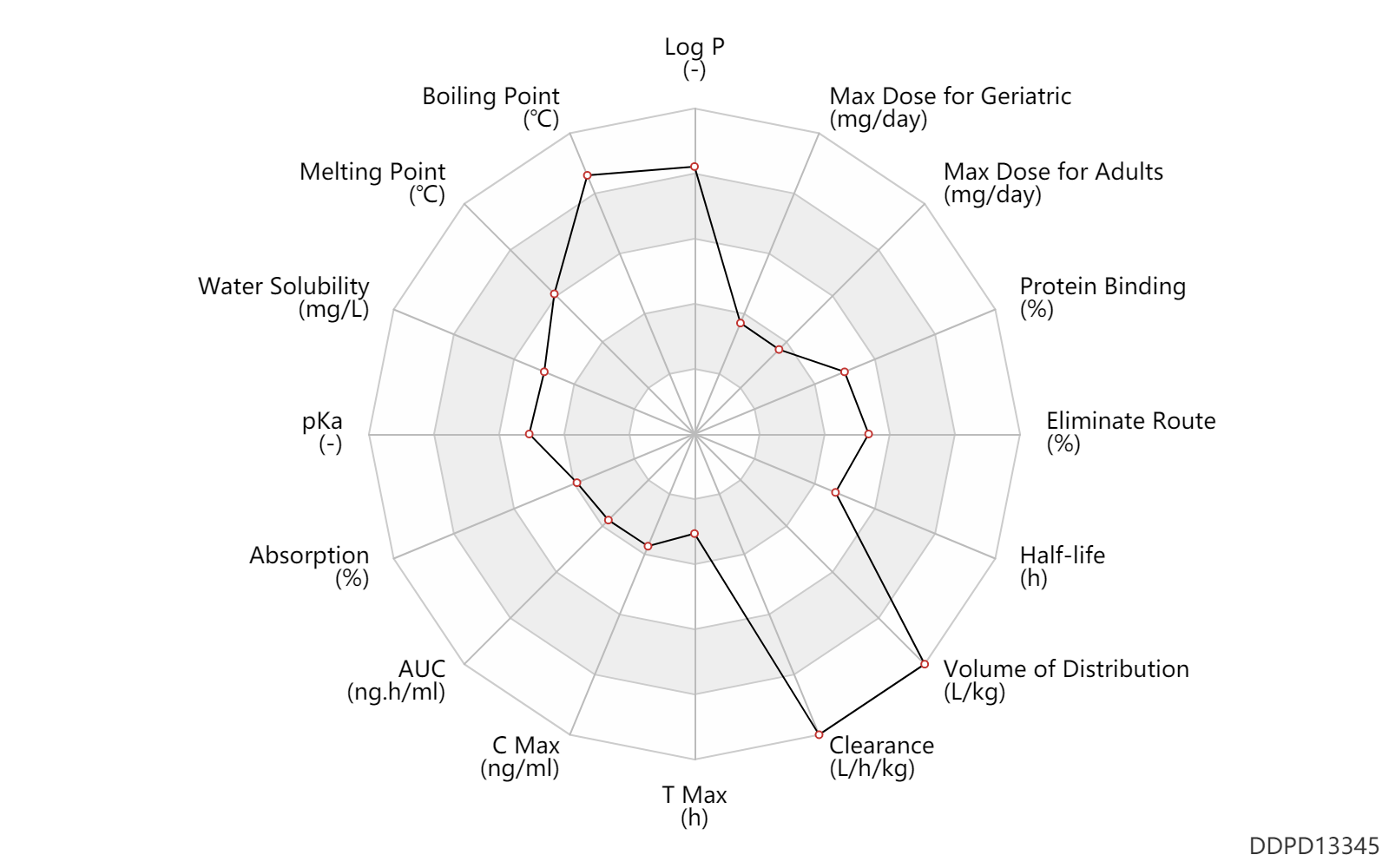
| Log P | 5.86 | - | 5.86 | - | Buckingham J. et al. Dictionary of Alkaloids. |
| Boiling Point | 899.3 | ℃ | 899.3 | ℃ | Product specifications |
| Melting Point | 214.0 | ℃ | 213-215 | ℃ | 'MSDS' |
| Water Solubility | 10000.0 | mg/L | 10 | mg/ml | 'MSDS' |
| pKa | 6.9 | - | 6.9 | - | Lemke T. and Williams D. Foye's Principles of Medicinal Chemistry. |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
| Absorption | 25.0 | % | 25 | % | PO, oral; | DRUGBANK |
| AUC | 0.39 | ng.h/ml | 0.39 | mcg.h/L | PO, oral; | DRUGBANK |
| C Max | 0.28 | ng/ml | 0.28 | mcg/L | PO, oral; | DRUGBANK |
| T Max | 0.46 | h | 0.46±0.17 | h | PO, oral; | DRUGBANK |
| Clearance | 2.7 | L/h/kg | 2.65 | L/h/kg | Total clearance; | DRUGBANK |
| Volume of Distribution | 52.0 | L/kg | 52.0 | L/kg | DRUGBANK | |
| Half-life | 3.5 | h | 3.5 | h | elimination half-life; | DRUGBANK | Half-life | 13.0 | h | 13 | h | terminal half-life; | DRUGBANK |
| Eliminate Route | 85.0 | % | >85 | % | Bile excretion; | DRUGBANK | Eliminate Route | 5.0 | % | 5 | % | Urinary excretion; | DRUGBANK |
| Protein Binding | 68.0 | % | 68 | % | plasma proteins; | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for adults | 3.0 | mg/day | 3 | mg/day | PO, oral | Ergoloid Mesylates | ergoloid mesylates | PDR |
| Max dose for adults | 9.0 | mg/day | 9 | mg/day | PO, oral | Ergoloid Mesylates | ergoloid mesylates | PDR |
| Max dose for geriatric | 3.0 | mg/day | 3 | mg/day | PO, oral | Ergoloid Mesylates | ergoloid mesylates | PDR |
| Max dose for geriatric | 9.0 | mg/day | 9 | mg/day | PO, oral | Ergoloid Mesylates | ergoloid mesylates | PDR |