Basic Information
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
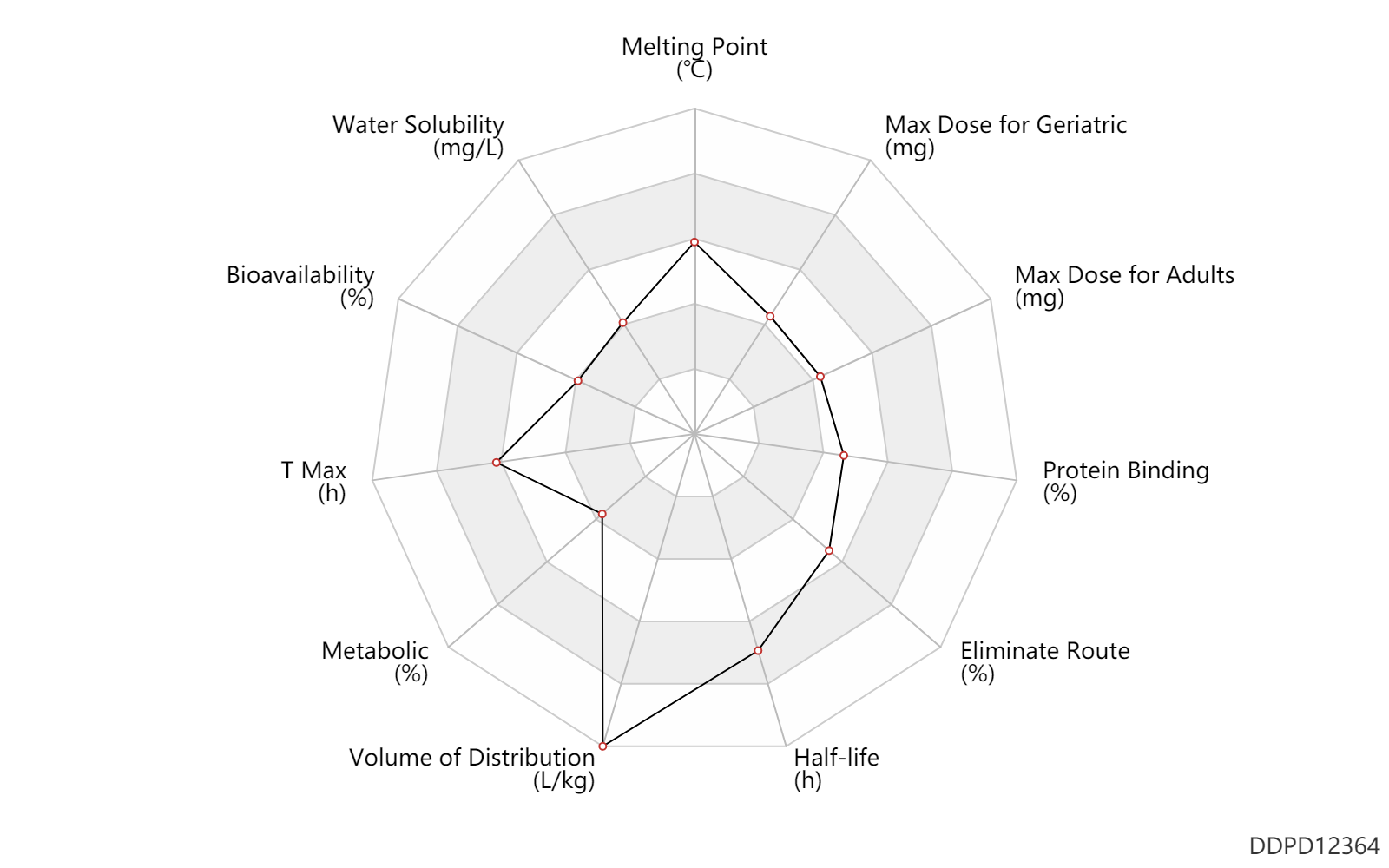
| Melting Point | 206.0 | ℃ | 200-212 | ℃ | Vincent, et al. Crystalline forms of a Factor Xa inhibitor. Patent application number WO 2012031017 A1 |
| Water Solubility | 2600.0 | mg/L | 2.5-2.7 | mg/ml | Vincent, et al. Crystalline forms of a Factor Xa inhibitor. Patent application number WO 2012031017 A1 |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
| Bioavailability | 34.0 | % | 34 | % | PO, oral; | DRUGBANK |
| T Max | 3.5 | h | 3-4 | h | PO, oral; normal,healthy; | DRUGBANK |
| Metabolic | 1.0 | % | <1 | % | Liver metabolism; | DRUGBANK |
| Volume of Distribution | 32.0 | L/kg | 32.0 | L/kg | Apparent volume of distribution; | DRUGBANK |
| Half-life | 23.0 | h | 19-27 | h | DRUGBANK | |
| Eliminate Route | 85.0 | % | 85 | % | Faeces excretion; | DRUGBANK | Eliminate Route | 11.0 | % | 11 | % | Urinary excretion; | DRUGBANK |
| Protein Binding | 60.0 | % | ~60 | % | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for adults | 160.0 | mg | 160 | mg | PO, oral | Bevyxxa | betrixaban | PDR |
| Max dose for geriatric | 160.0 | mg | 160 | mg | PO, oral | Bevyxxa | betrixaban | PDR |