Basic Information
Experimental Physicochemical Property
Not Available
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
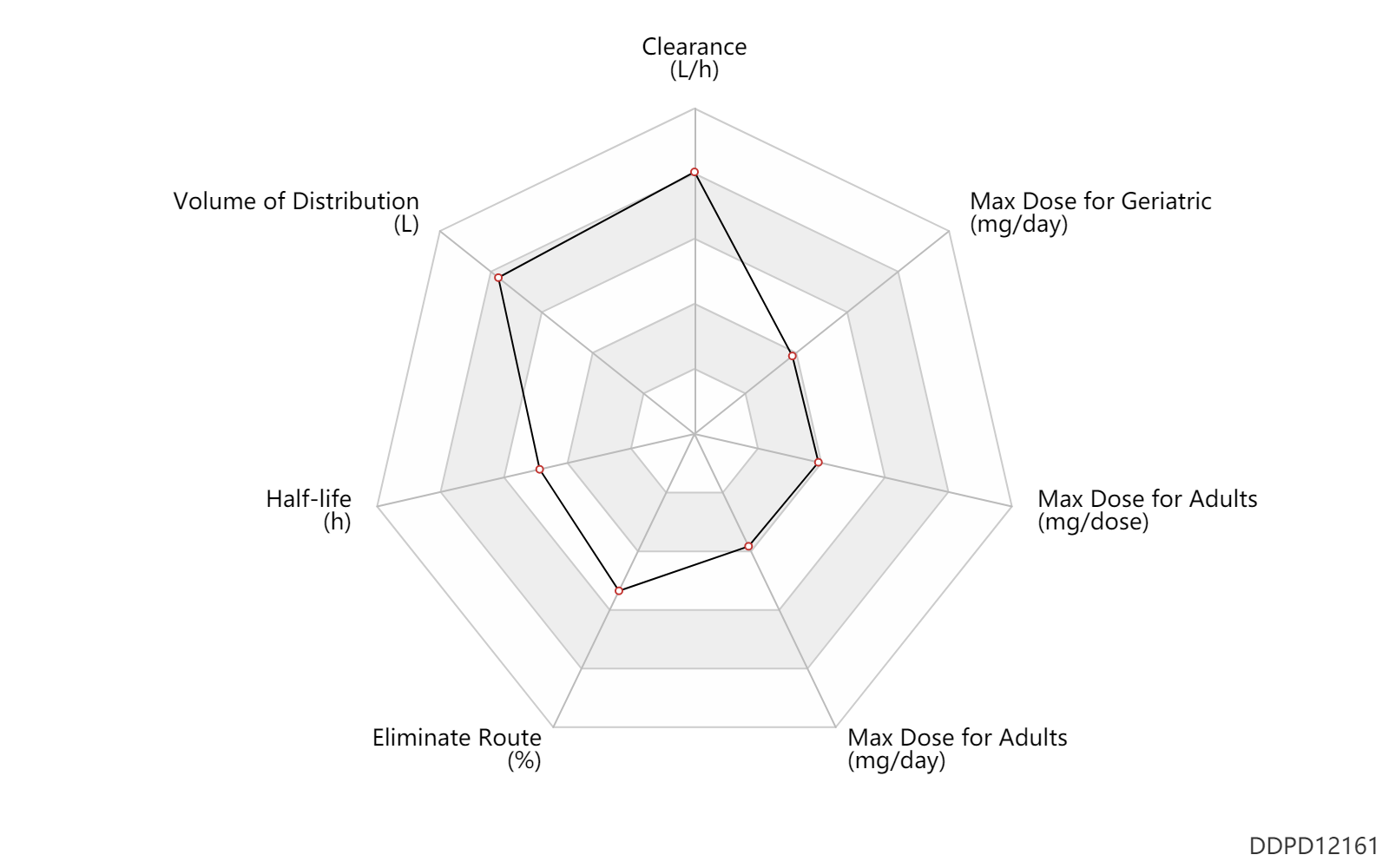
| Clearance | 47.0 | L/h | 47.0 | L/h | Average clearance; Huntington's disease; | DRUGBANK | Clearance | 70.0 | L/h | 70.0 | L/h | Average clearance; Huntington's disease; | DRUGBANK |
| Volume of Distribution | 500.0 | L | ~500 | L | Average volume of distribution; | DRUGBANK | Volume of Distribution | 730.0 | L | ~730 | L | Average volume of distribution; | DRUGBANK |
| Half-life | 9.5 | h | 9-10 | h | DRUGBANK | |
| Eliminate Route | 80.5 | % | ~75-86 | % | Urinary excretion; normal,healthy; human, homo sapiens; | DRUGBANK | Eliminate Route | 9.5 | % | 8-11 | % | Faeces excretion; normal,healthy; human, homo sapiens; | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for adults | 48.0 | mg/day | 48 | mg/day | PO, oral | Austedo | deutetrabenazine | PDR |
| Max dose for adults | 18.0 | mg/dose | 18 | mg/dose | PO, oral | Austedo | deutetrabenazine | PDR |
| Max dose for adults | 36.0 | mg/day | 36 | mg/day | PO, oral | Austedo | deutetrabenazine | PDR |
| Max dose for geriatric | 48.0 | mg/day | 48 | mg/day | PO, oral | Austedo | deutetrabenazine | PDR |
| Max dose for geriatric | 18.0 | mg/dose | 18 | mg/dose | PO, oral | Austedo | deutetrabenazine | PDR |
| Max dose for geriatric | 36.0 | mg/day | 36 | mg/day | PO, oral | Austedo | deutetrabenazine | PDR |