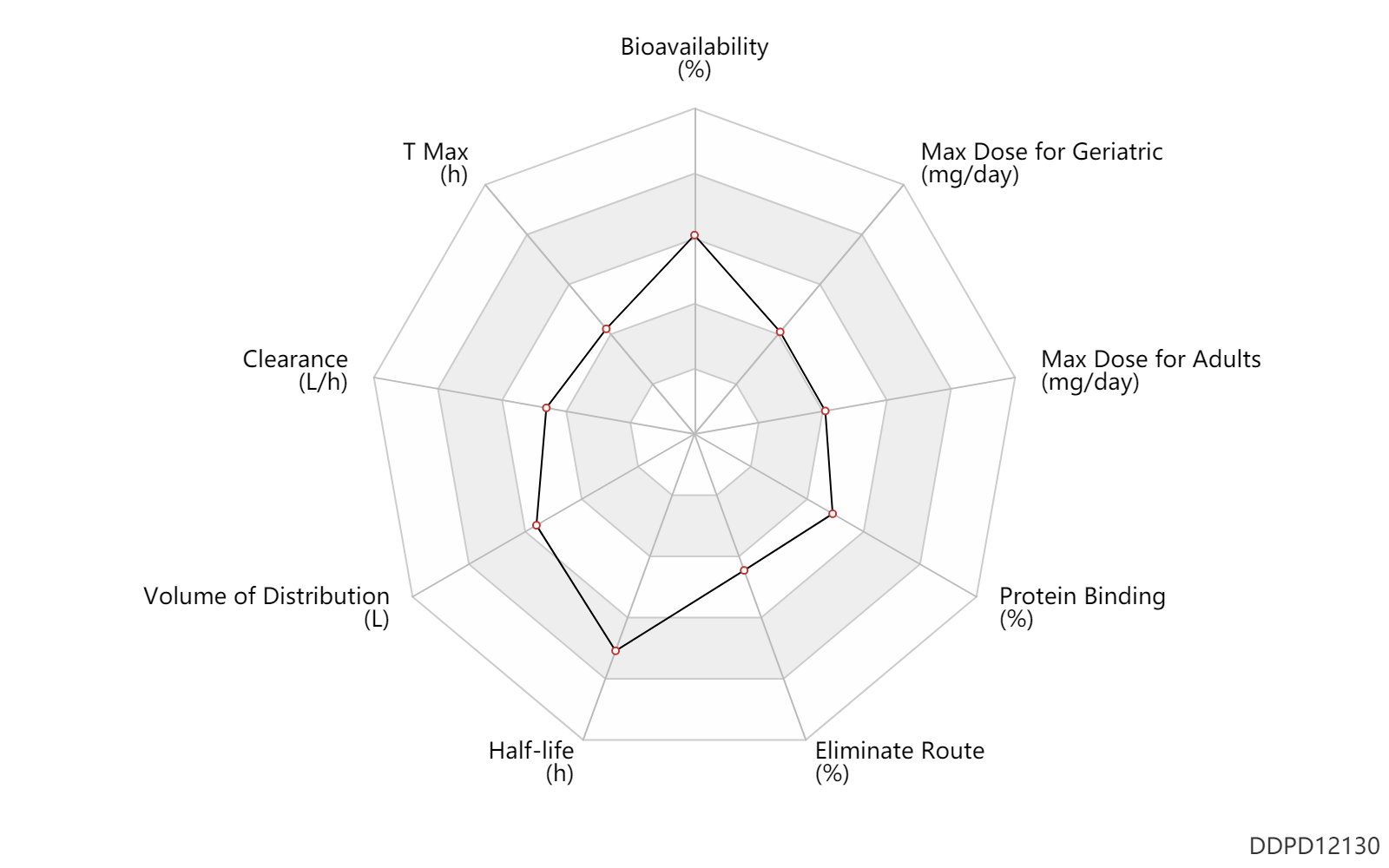
| Property Name |
Property Value |
Unit |
Raw Value |
Raw Unit |
Annotation |
Reference |
| Bioavailability |
81.0 |
% |
81 |
% |
PO, oral; |
DRUGBANK |
| T Max |
1.2 |
h |
1.2(0.5-4) |
h |
Oral single dose; |
DRUGBANK |
T Max |
2.0 |
h |
2(0.53-23) |
h |
Oral multiple dose; |
DRUGBANK |
| Clearance |
11.0 |
L/h |
11.0 |
L/h |
Average clearance; Oral single dose; |
DRUGBANK |
Clearance |
18.0 |
L/h |
18.0 |
L/h |
Average clearance; at steady state; |
DRUGBANK |
| Volume of Distribution |
305.0 |
L |
305.0 |
L |
Steady state volume of distribution; intravenous injection, IV; Single dose; |
DRUGBANK |
| Half-life |
24.0 |
h |
24 |
h |
elimination half-life; Oral single dose; |
DRUGBANK |
| Eliminate Route |
48.0 |
% |
48 |
% |
Urinary excretion; Oral single dose; |
DRUGBANK |
Eliminate Route |
41.0 |
% |
41 |
% |
Faeces excretion; Oral single dose; |
DRUGBANK |
Eliminate Route |
0.48 |
% |
<0.48 |
% |
Urinary excretion; Oral single dose; Unchanged drug; |
DRUGBANK |
Eliminate Route |
3.7 |
% |
~3.69 |
% |
Faeces excretion; Oral single dose; Unchanged drug; |
DRUGBANK |
| Protein Binding |
66.0 |
% |
66 |
% |
plasma proteins; high protein binding; |
DRUGBANK |