Basic Information
Experimental Physicochemical Property
Not Available
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
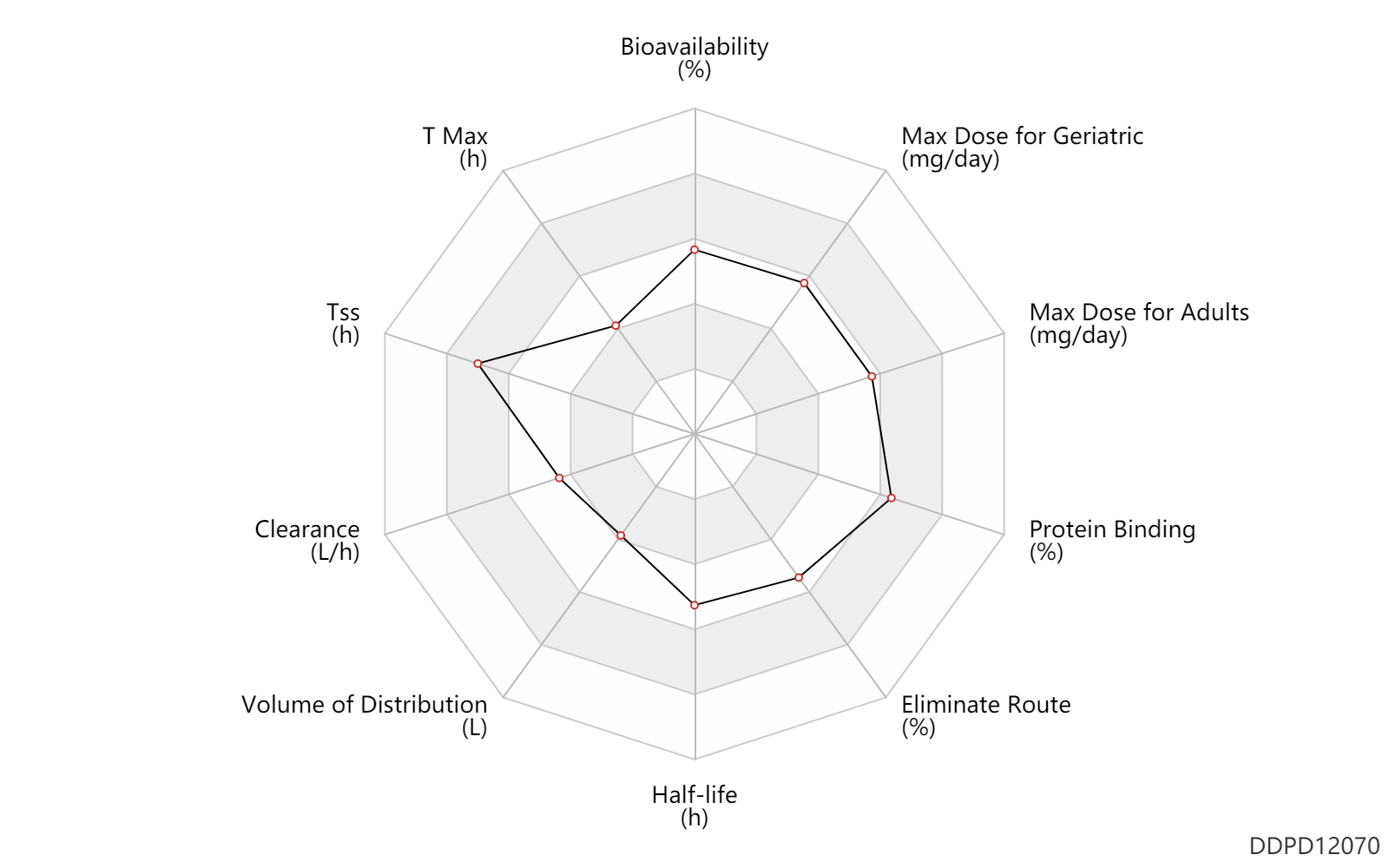
| Bioavailability | 94.0 | % | 94 | % | DRUGBANK | Bioavailability | 35.0 | % | 35 | % | hematopoietic stem cell transplant; | DRUGBANK | Bioavailability | 85.0 | % | 85 | % | Drug combination; hematopoietic stem cell transplant; | DRUGBANK |
| T Max | 1.5 | h | 0.75-2.25 | h | DRUGBANK | |
| Tss | 228.0 | h | 9-10 | day | DRUGBANK | |
| Clearance | 11.3 | L/h | 11.25 | L/h | Average clearance; normal,healthy; | DRUGBANK |
| Volume of Distribution | 45.5 | L | 45.5 | L | Steady state volume of distribution; | DRUGBANK |
| Half-life | 12.0 | h | 12 | h | terminal half-life; intravenous injection, IV; | DRUGBANK |
| Eliminate Route | 93.0 | % | 93 | % | Faeces excretion; | DRUGBANK | Eliminate Route | 2.0 | % | <2 | % | Urinary excretion; | DRUGBANK |
| Protein Binding | 99.0 | % | 99 | % | plasma proteins; high protein binding; | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for adults | 480.0 | mg/day | 480 | mg/day | PO, oral;intravenous injection, IV; | Prevymis | letermovir | PDR |
| Max dose for geriatric | 480.0 | mg/day | 480 | mg/day | PO, oral;intravenous injection, IV; | Prevymis | letermovir | PDR |