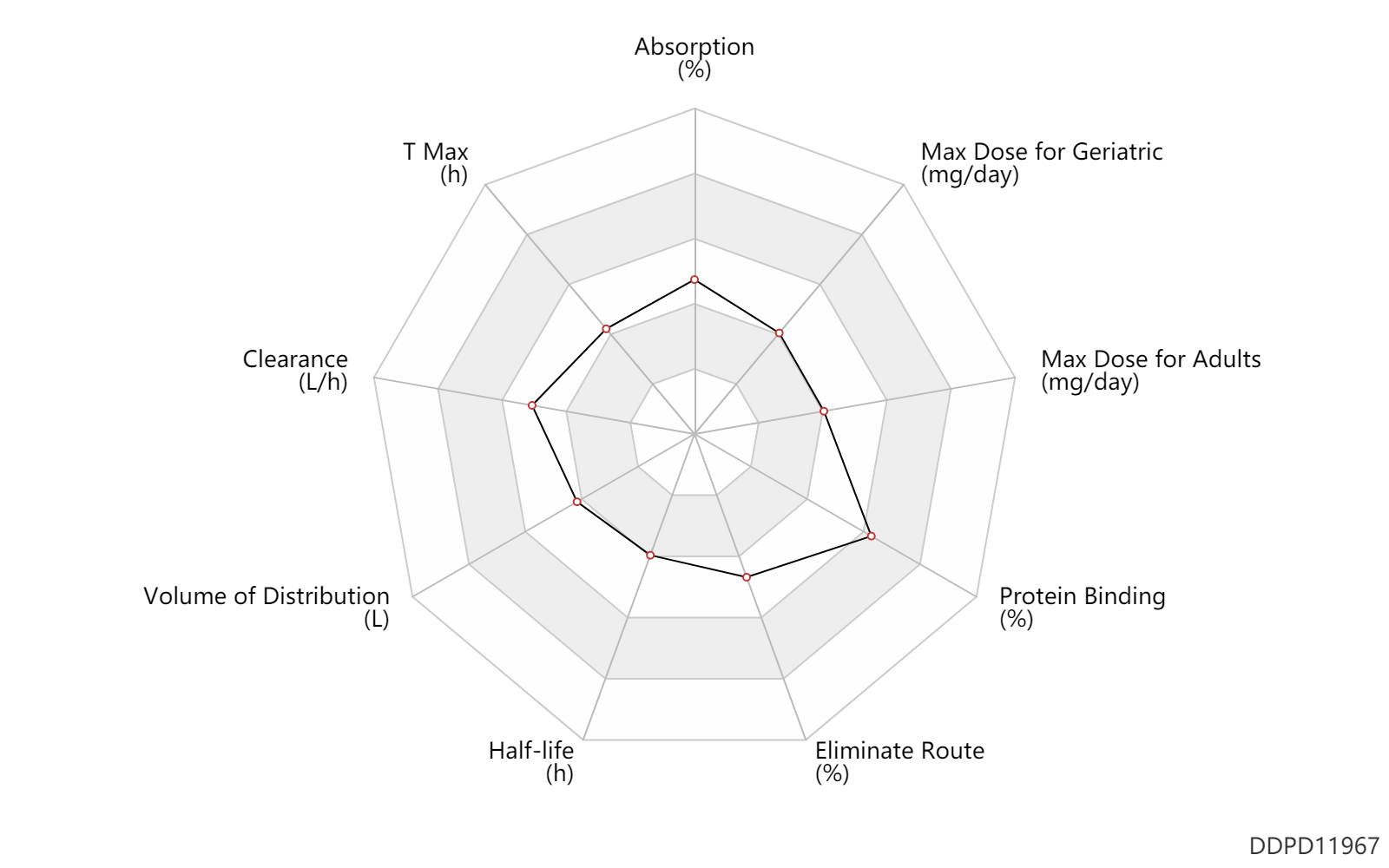
| Property Name |
Property Value |
Unit |
Raw Value |
Raw Unit |
Annotation |
Reference |
| Absorption |
50.0 |
% |
>50 |
% |
PO, oral; |
DRUGBANK |
| T Max |
1.6 |
h |
1.6 |
h |
PO, oral; |
DRUGBANK |
| Clearance |
20.2 |
L/h |
20.2 |
L/h |
|
DRUGBANK |
| Volume of Distribution |
92.0 |
L |
92.0 |
L |
Apparent volume of distribution; |
DRUGBANK |
| Half-life |
3.5 |
h |
3.5 |
h |
terminal half-life; |
DRUGBANK |
| Eliminate Route |
62.0 |
% |
62 |
% |
Faeces excretion; Oral single dose; normal,healthy; human, homo sapiens; |
DRUGBANK |
Eliminate Route |
31.0 |
% |
31 |
% |
Urinary excretion; Oral single dose; normal,healthy; human, homo sapiens; |
DRUGBANK |
Eliminate Route |
19.8 |
% |
19.84 |
% |
Faeces excretion; Oral single dose; human, homo sapiens; Unchanged drug; |
DRUGBANK |
Eliminate Route |
2.0 |
% |
2.01 |
% |
Urinary excretion; Oral single dose; human, homo sapiens; Unchanged drug; |
DRUGBANK |
| Protein Binding |
97.0 |
% |
97 |
% |
plasma proteins; human, homo sapiens; |
DRUGBANK |