Basic Information
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
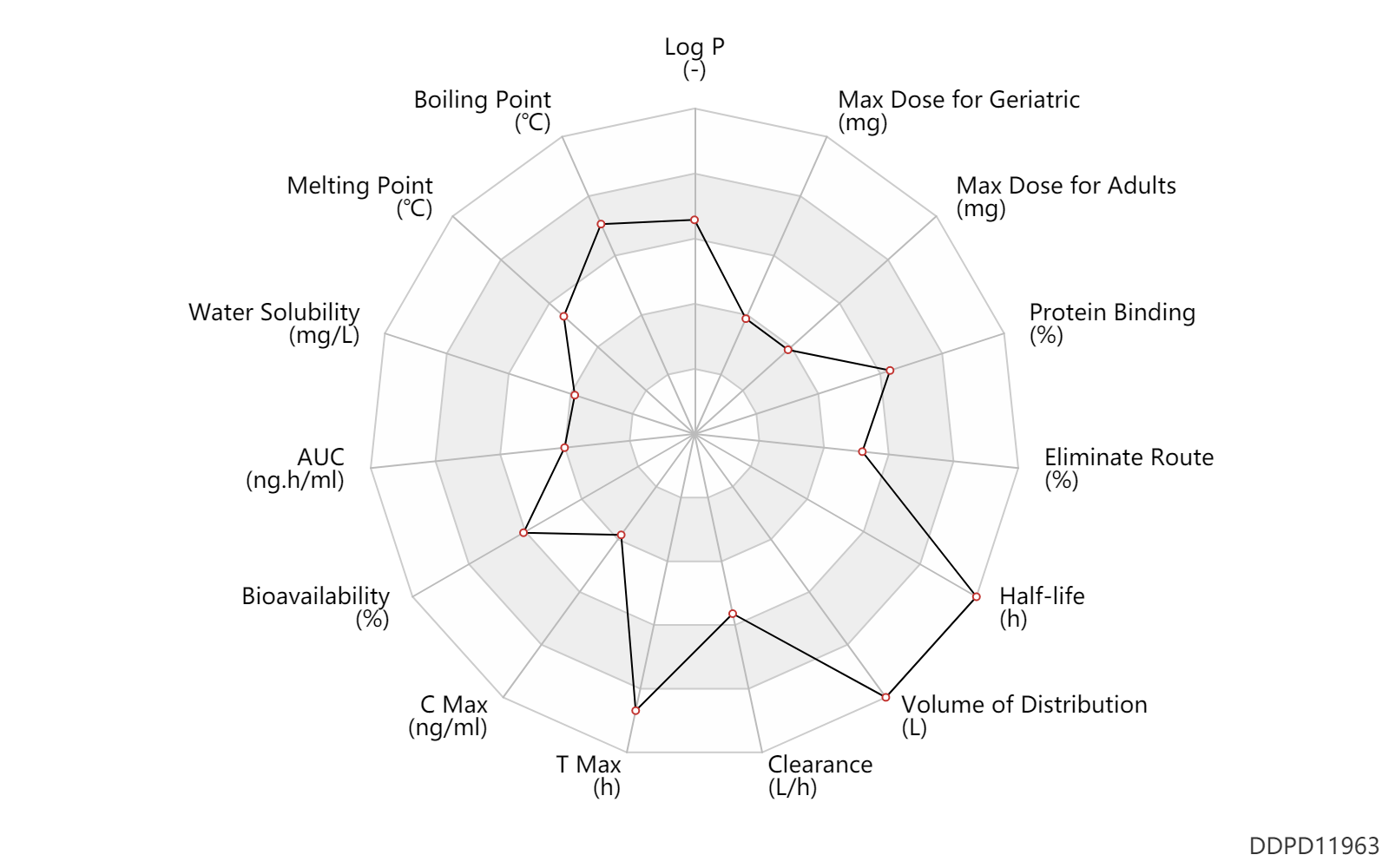
| Log P | 3.92 | - | 3.92 | - | 'MSDS' |
| Boiling Point | 665.7 | ℃ | 665.7 | ℃ | 'MSDS' |
| Melting Point | 185.5 | ℃ | 184-187 | ℃ | Williams J., et al. (2014). Canc. Treatment Rev. 40, 917. |
| Water Solubility | 1000.0 | mg/L | <1 | mg/ml | 'MSDS' |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
| AUC | 2213.0 | ng.h/ml | 2213.0 | ng.h/ml | PO, oral; | DRUGBANK |
| Bioavailability | 80.0 | % | 80 | % | PO, oral; | DRUGBANK |
| C Max | 104.0 | ng/ml | 104 | ng/ml | PO, oral; | DRUGBANK |
| T Max | 6.0 | h | 6 | h | PO, oral; | DRUGBANK |
| Clearance | 27.6 | L/h | 27.6 | L/h | apparent clearance; | DRUGBANK |
| Volume of Distribution | 2415.0 | L | 2415.0 | L | DRUGBANK | |
| Half-life | 70.0 | h | 70 | h | DRUGBANK | |
| Eliminate Route | 79.0 | % | 79 | % | Faeces excretion; | DRUGBANK | Eliminate Route | 3.0 | % | 3 | % | Urinary excretion; | DRUGBANK |
| Protein Binding | 98.0 | % | 98 | % | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Frequency | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Max dose for adults | 45.0 | mg | 45 | mg | PO, oral | qd | Vizimpro | dacomitinib | PDR |
| Max dose for geriatric | 45.0 | mg | 45 | mg | PO, oral | qd | Vizimpro | dacomitinib | PDR |