Basic Information
Experimental Physicochemical Property
Not Available
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Factor | Reference |
|---|---|---|---|---|---|---|---|
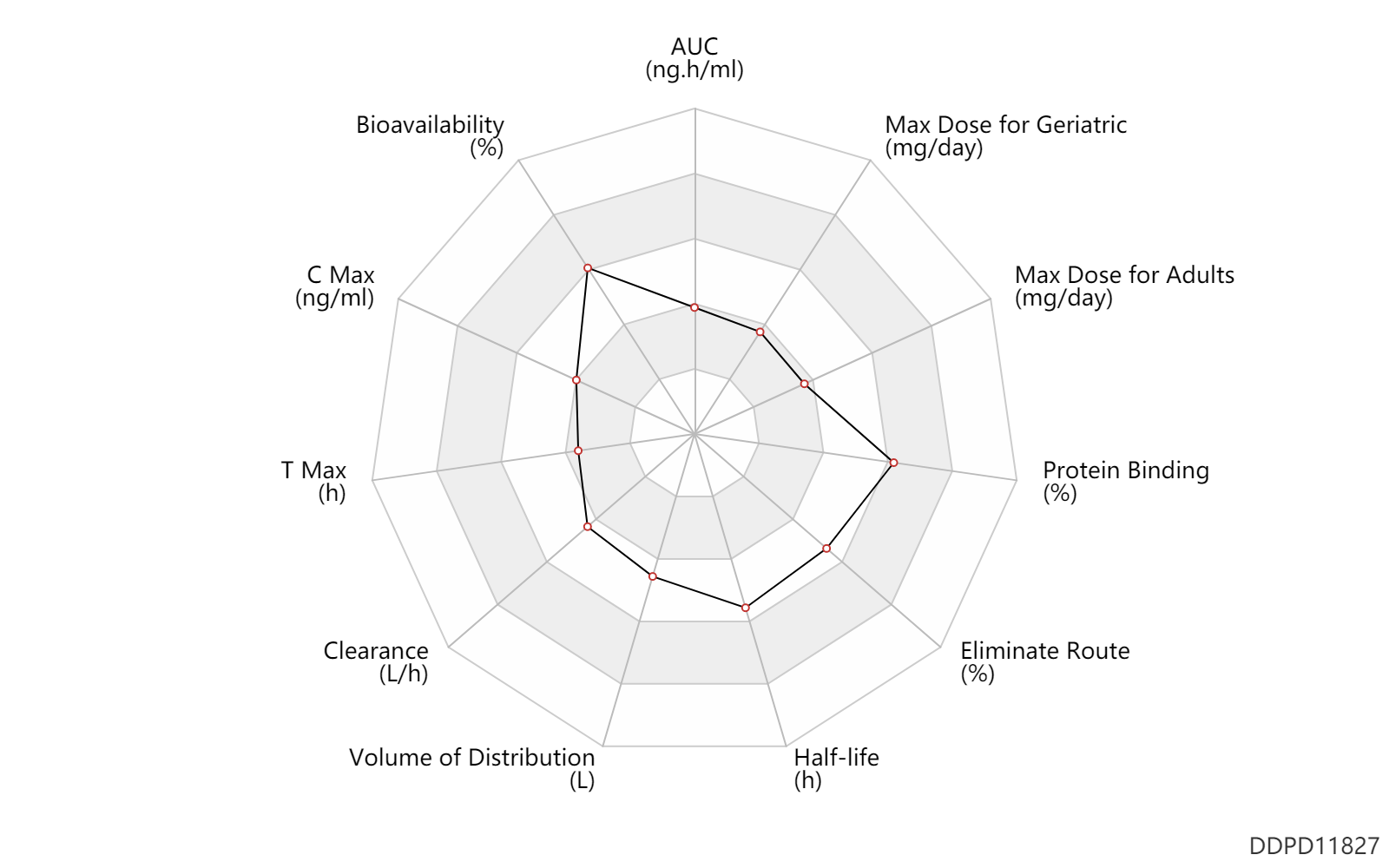
| AUC | 1193.0 | ng.h/ml | 1193.0 | ng.h/ml | PO, oral; | DRUGBANK | |
| Bioavailability | 80.0 | % | 70-90 | % | PO, oral; | DRUGBANK | |
| C Max | 268.0 | ng/ml | 268 | ng/ml | PO, oral; | DRUGBANK | |
| T Max | 1.0 | h | 0.5-1.5 | h | PO, oral; | DRUGBANK | |
| Clearance | 10.7 | L/h | 178.7 | ml/min | Plasma clearance; PO, oral; | DRUGBANK | Clearance | 11.2 | L/h | 187.2 | ml/min | Total clearance; intravenous injection, IV; | DRUGBANK |
| Volume of Distribution | 215.3 | L | 215.3 | L | Apparent volume of distribution; PO, oral; | DRUGBANK | Volume of Distribution | 85.5 | L | 85.53 | L | Steady state volume of distribution; intravenous injection, IV; | DRUGBANK |
| Half-life | 14.0 | h | 11-17 | h | elimination half-life; | DRUGBANK | |
| Eliminate Route | 50.0 | % | 50 | % | Urinary excretion; | DRUGBANK | Eliminate Route | 41.0 | % | 41 | % | Faeces excretion; | DRUGBANK |
| Protein Binding | 95.0 | % | 94-96 | % | plasma proteins; | Plasma Concentration → ; | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for adults | 15.0 | mg/day | 15 | mg/day | PO, oral | Steglatro | ertugliflozin | PDR |
| Max dose for geriatric | 15.0 | mg/day | 15 | mg/day | PO, oral | Steglatro | ertugliflozin | PDR |