Basic Information
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
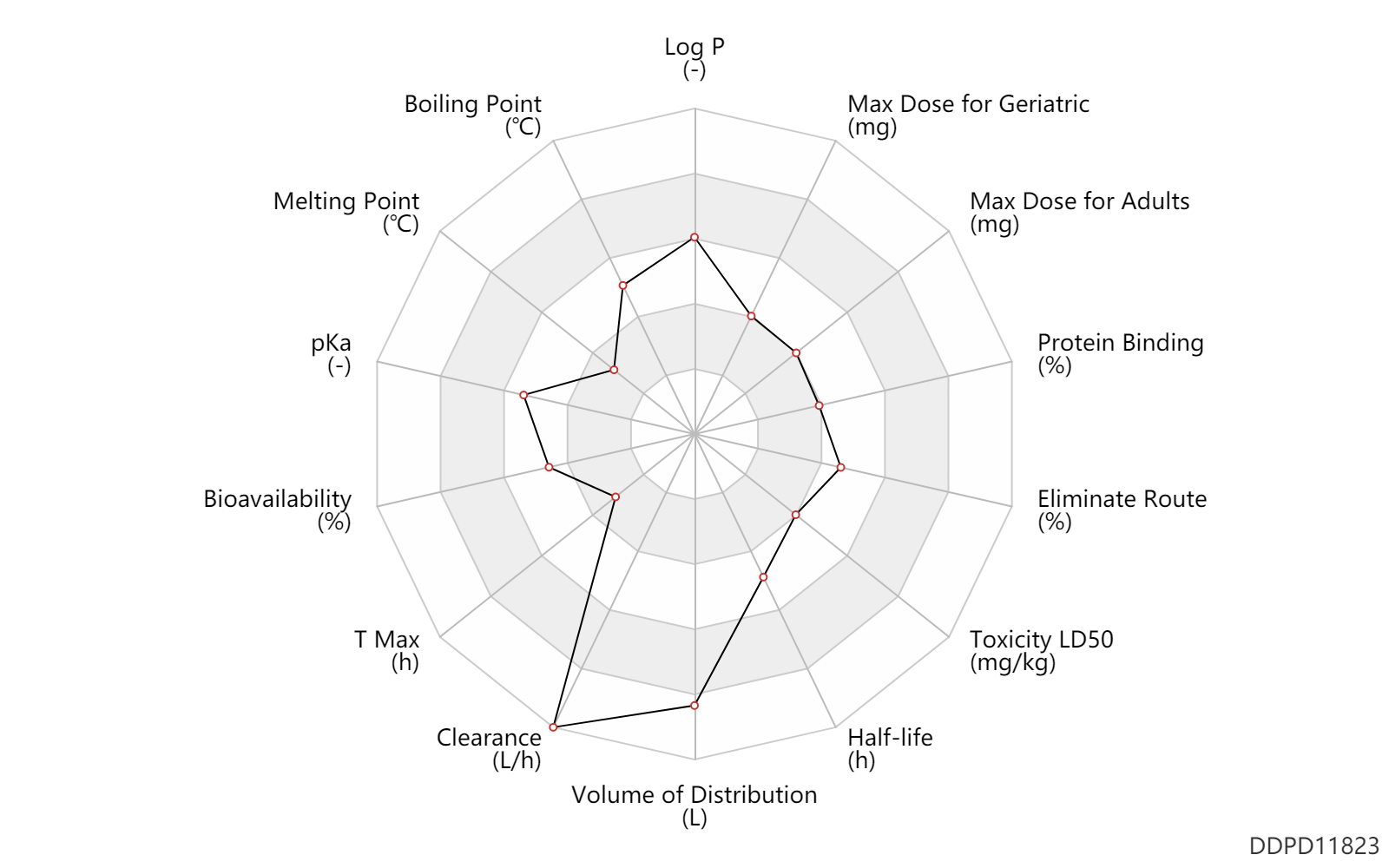
| Log P | 3.2887 | - | 3.2887 | - | https://www.chemsrc.com/en/cas/33643-46-8_146615.html |
| Boiling Point | 363.8 | ℃ | 363.8 | ℃ | https://www.chemsrc.com/en/cas/33643-46-8_146615.html |
| Melting Point | 92.5 | ℃ | 92.5 | ℃ | http://pharmacycode.com/Esketamine.html |
| pKa | 7.5 | - | 7.5 | - | https://edoc.unibas.ch/1310/1/20110314_1408_DissCB_e_version.pdf |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
| Bioavailability | 48.0 | % | 48 | % | nasal spray; | DRUGBANK |
| T Max | 0.50 | h | 20-40 | min | nasal spray; | DRUGBANK |
| Clearance | 89.0 | L/h | 89.0 | L/h | Average clearance; intravenous injection, IV; | DRUGBANK |
| Volume of Distribution | 709.0 | L | 709.0 | L | Apparent volume of distribution; intravenous injection, IV; | DRUGBANK |
| Half-life | 9.5 | h | 7-12 | h | terminal half-life; | DRUGBANK |
| Toxicity LD50 | 447.0 | mg/kg | 447.0 | mg/kg | PO, oral; Rattus, Rat; | DRUGBANK |
| Eliminate Route | 78.0 | % | ≥78 | % | Urinary excretion; PO, oral; intravenous injection, IV; | DRUGBANK | Eliminate Route | 2.0 | % | ≤2 | % | Faeces excretion; PO, oral; intravenous injection, IV; | DRUGBANK | Eliminate Route | 1.0 | % | <1 | % | Unchanged drug; | DRUGBANK |
| Protein Binding | 44.0 | % | 43-45 | % | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Frequency | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Max dose for infants | 0.0 | 0 | Spravato | esketamine hydrochloride | PDR | ||||
| Max dose for adults | 84.0 | mg | 84 | mg | intranasal | biw | Spravato | esketamine hydrochloride | PDR |
| Max dose for geriatric | 84.0 | mg | 84 | mg | intranasal | biw | Spravato | esketamine hydrochloride | PDR |