Basic Information
Experimental Physicochemical Property
Not Available
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
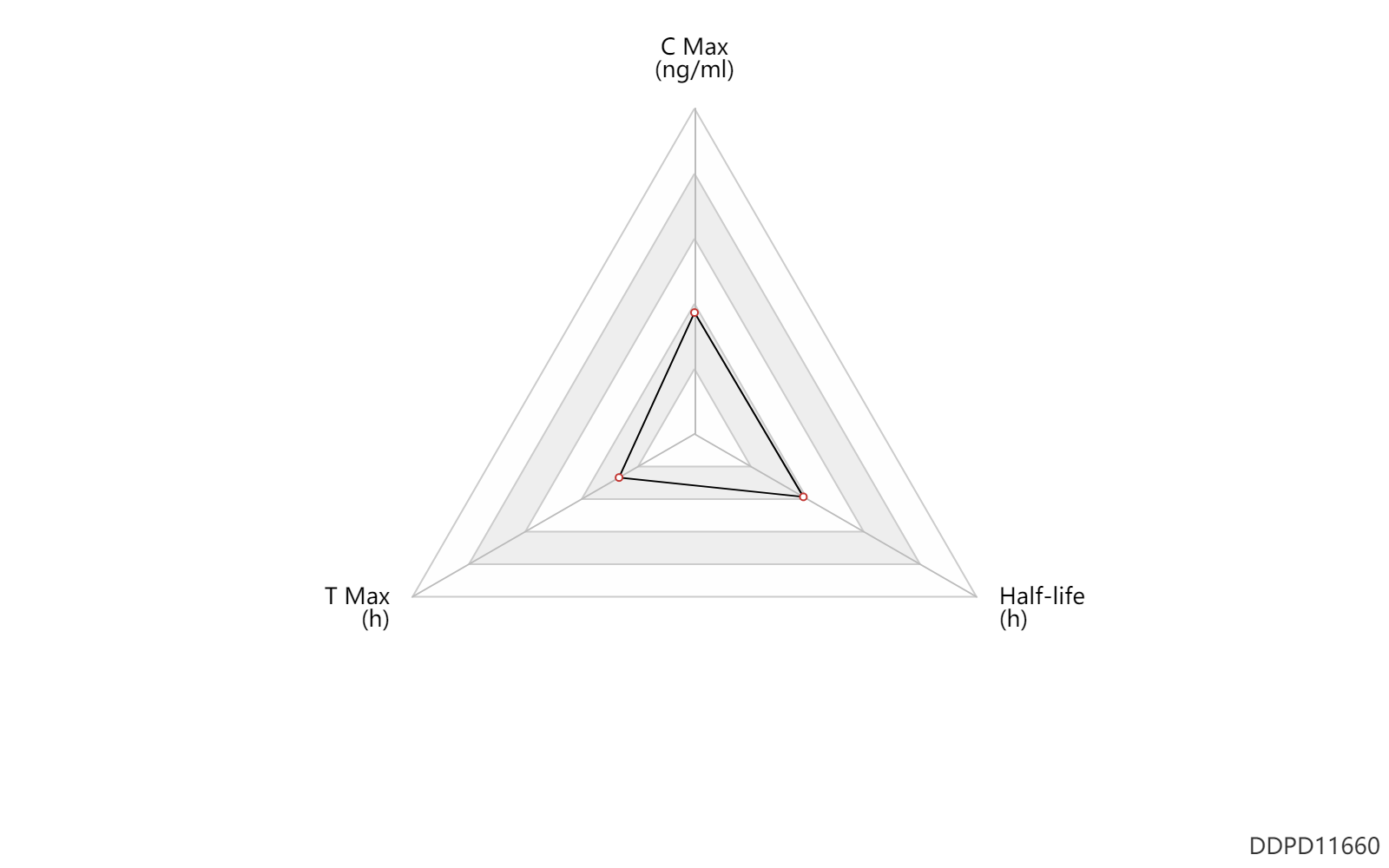
| C Max | 0.0591 | ng/ml | 59.1 | pg/ml | normal,healthy; Derivative; | DRUGBANK |
| T Max | 0.0833 | h | 5 | min | normal,healthy; Derivative; | DRUGBANK |
| Half-life | 1.8 | h | 1.8 | h | rabbit; | DRUGBANK | Half-life | 2.1 | h | 2.1 | h | rabbit; | DRUGBANK | Half-life | 4.6 | h | 4.6 | h | rabbit; | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for neonates | 0.0 | 0 | Vyzulta | latanoprostene bunod | PDR | |||
| Max dose for infants | 0.0 | 0 | Vyzulta | latanoprostene bunod | PDR | |||
| Max dose for children | 0.0 | 0 | Vyzulta | latanoprostene bunod | PDR | |||
| Max dose for adolescents | 1.0 | drop/day | 1 | drop/day | ophthalmic administration | Vyzulta | latanoprostene bunod | PDR |
| Max dose for adolescents | 0.0 | 0 | Vyzulta | latanoprostene bunod | PDR | |||
| Max dose for adults | 1.0 | drop/day | 1 | drop/day | ophthalmic administration | Vyzulta | latanoprostene bunod | PDR |
| Max dose for geriatric | 1.0 | drop/day | 1 | drop/day | ophthalmic administration | Vyzulta | latanoprostene bunod | PDR |