Basic Information
| Drug ID | DDPD09299 |

|
| Drug Name | Tenofovir alafenamide | |
| Molecular Weight | 476.474 | |
| Molecular Formula | C21H29N6O5P | |
| CAS Number | 379270-37-8 | |
| SMILES | CC(C)OC(=O)[C@H](C)N[P@](=O)(CO[C@H](C)CN1C=NC2=C(N)N=CN=C12)OC1=CC=CC=C1 | |
| External Links | ||
| DRUGBANK | DB09299 | |
| PubChem Compound | 9574768 | |
| PDR | 23981 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
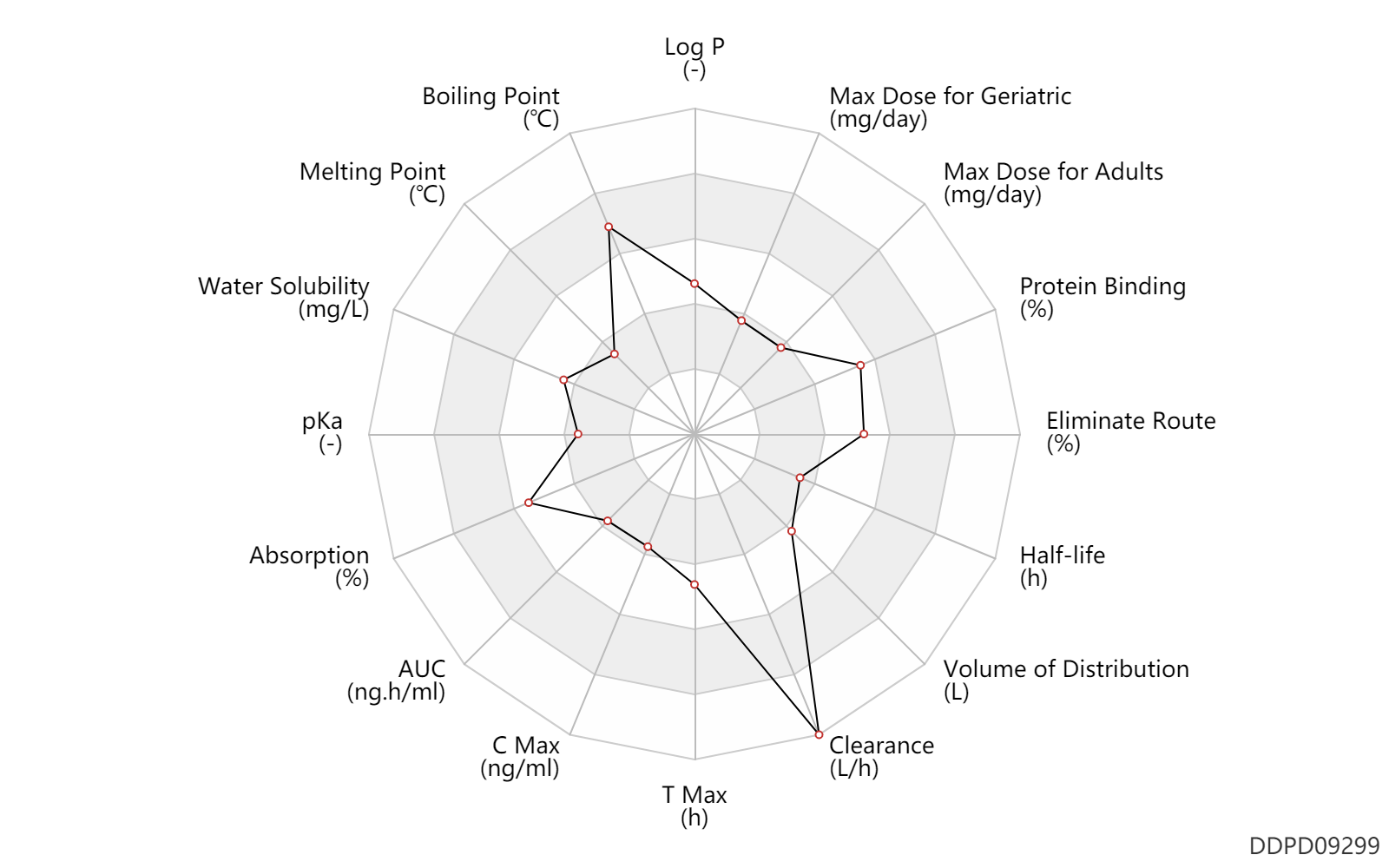
| Log P | 1.6 | - | 1.6 | - | Product monograph. Gilead Sciences Canada |
| Boiling Point | 640.0 | ℃ | 640 | ℃ | 'MSDS' |
| Melting Point | 105.5 | ℃ | 104-107 | ℃ | 'MSDS' |
| Water Solubility | 4860.0 | mg/L | 4.86 | mg/ml | Product monograph. Gilead Sciences Canada |
| pKa | 3.96 | - | 3.96 | - | Product monograph. Gilead Sciences Canada |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
| Absorption | 73.0 | % | 73 | % | Oral single dose; | DRUGBANK |
| AUC | 270.0 | ng.h/ml | 270.0 | ng.h/ml | Oral single dose; | DRUGBANK |
| C Max | 16.0 | ng/ml | 16 | ng/ml | Oral single dose; | DRUGBANK |
| T Max | 2.0 | h | 2 | h | Oral single dose; | DRUGBANK |
| Clearance | 117.0 | L/h | 117.0 | L/h | DRUGBANK | Clearance | 61.7 | L/h | 61.7 | L/h | severe renal function; patients; | DRUGBANK |
| Volume of Distribution | 100.0 | L | >100 | L | DRUGBANK | |
| Half-life | 0.51 | h | 0.51 | h | DRUGBANK | |
| Eliminate Route | 47.0 | % | 47 | % | Bile excretion; | DRUGBANK | Eliminate Route | 36.0 | % | 36 | % | Urinary excretion; | DRUGBANK |
| Protein Binding | 80.0 | % | ~80 | % | plasma proteins; in vivo; | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for adults | 25.0 | mg/day | 25 | mg/day | PO, oral | Vemlidy | tenofovir alafenamide | PDR |
| Max dose for geriatric | 25.0 | mg/day | 25 | mg/day | PO, oral | Vemlidy | tenofovir alafenamide | PDR |