Basic Information
| Drug ID | DDPD09291 |

|
| Drug Name | Rolapitant | |
| Molecular Weight | 500.485 | |
| Molecular Formula | C25H26F6N2O2 | |
| CAS Number | 552292-08-7 | |
| SMILES | C[C@@H](OC[C@]1(CC[C@]2(CCC(=O)N2)CN1)C1=CC=CC=C1)C1=CC(=CC(=C1)C(F)(F)F)C(F)(F)F | |
| External Links | ||
| DRUGBANK | DB09291 | |
| PubChem Compound | 10311306 | |
| PDR | 3808 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
Not Available
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
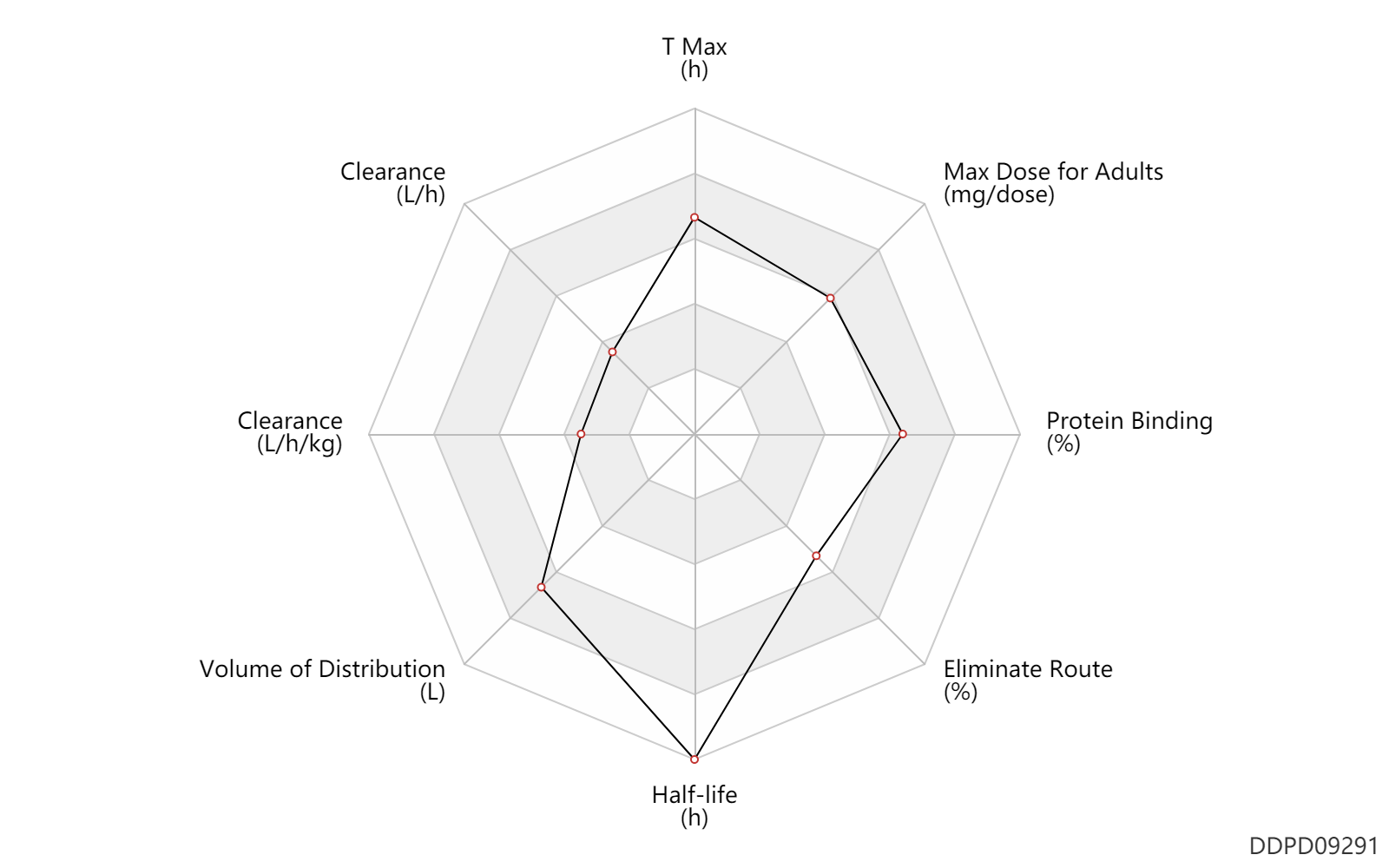
| T Max | 4.0 | h | 4 | h | DRUGBANK | |
| Clearance | 0.96 | L/h | 0.96 | L/h | DRUGBANK | Clearance | 0.0228 | L/h/kg | 0.38 | ml/min/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Volume of Distribution | 460.0 | L | 460.0 | L | DRUGBANK | |
| Half-life | 176.0 | h | 169-183 | h | terminal half-life; | DRUGBANK | Half-life | 148.5 | h | 148.5 | h | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Eliminate Route | 14.2 | % | 14.2 | % | Urinary excretion; | DRUGBANK | Eliminate Route | 73.0 | % | 73 | % | Faeces excretion; | DRUGBANK |
| Protein Binding | 99.8 | % | 99.8 | % | plasma proteins; human, homo sapiens; | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for adults | 180.0 | mg/dose | 180 | mg/dose | PO, oral; on day 1 of the chemotherapy regimen | Varubi | rolapitant | PDR |
| Max dose for adults | 166.5 | mg/dose | 166.5 | mg/dose | intravenous injection, IV; on day 1 of the chemotherapy regimen | Varubi | rolapitant | PDR |
| Max dose for geriatric | 180.0 | mg/dose | 180 | mg/dose | PO, oral; on day 1 of the chemotherapy regimen | Varubi | rolapitant | PDR |
| Max dose for geriatric | 166.5 | mg/dose | 166.5 | mg/dose | intravenous injection, IV; on day 1 of the chemotherapy regimen | Varubi | rolapitant | PDR |