Basic Information
| Drug ID | DDPD09132 |

|
| Drug Name | Gadoteric acid | |
| Molecular Weight | 558.65 | |
| Molecular Formula | C16H25GdN4O8 | |
| CAS Number | 72573-82-1 | |
| SMILES | [Gd+3].OC(=O)CN1CCN(CC([O-])=O)CCN(CC([O-])=O)CCN(CC([O-])=O)CC1 | |
| External Links | ||
| DRUGBANK | DB09132 | |
| PubChem Compound | 158536 | |
| PDR | 3146 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
Not Available
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
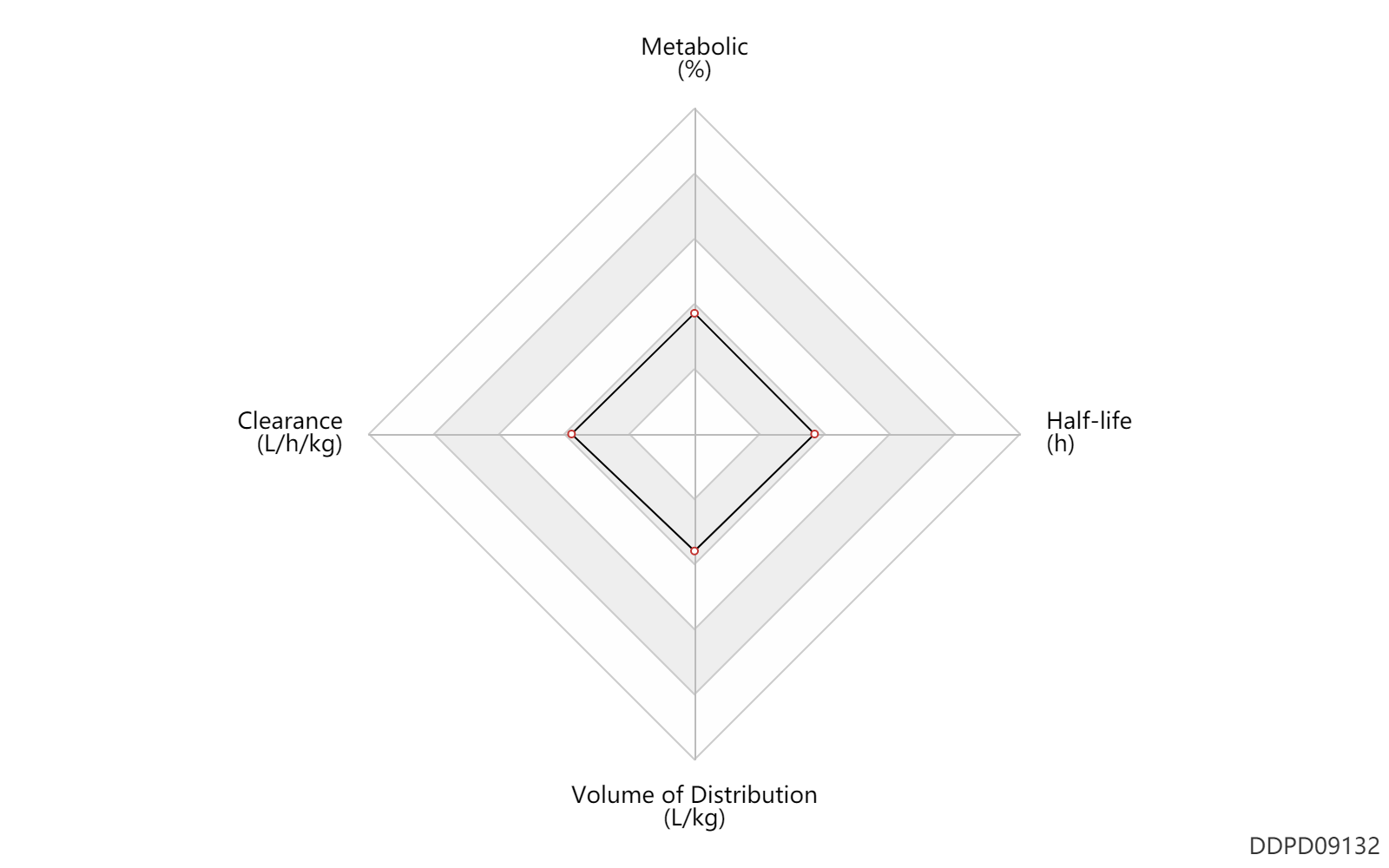
| Metabolic | 0 | % | 0 | % | DRUGBANK | |
| Clearance | 0.0762 | L/h/kg | 1.27 | ml/min/kg | Renal clearance; normal,healthy; Female, women; | DRUGBANK | Clearance | 0.10 | L/h/kg | 1.74 | ml/min/kg | Total clearance; normal,healthy; Female, women; | DRUGBANK | Clearance | 0.0840 | L/h/kg | 1.4 | ml/min/kg | Renal clearance; normal,healthy; Male, men; | DRUGBANK | Clearance | 0.0984 | L/h/kg | 1.64 | ml/min/kg | Total clearance; normal,healthy; Male, men; | DRUGBANK |
| Volume of Distribution | 0.18 | L/kg | 179.0 | ml/kg | at steady state; normal,healthy; Female, women; | DRUGBANK | Volume of Distribution | 0.21 | L/kg | 211.0 | ml/kg | at steady state; normal,healthy; Male, men; | DRUGBANK |
| Half-life | 1.4 | h | 1.4 | h | elimination half-life; Female, women; | DRUGBANK | Half-life | 2.0 | h | 2.0 | h | elimination half-life; Male, men; | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for neonates | 0.2 | ml/kg/dose | 0.2 | ml/kg/dose | intravenous injection, IV | Dotarem Injection | gadoterate meglumine | PDR |
| Max dose for neonates | 0.1 | mmol/kg/dose | 0.1 | mmol/kg/dose | intravenous injection, IV | Dotarem Injection | gadoterate meglumine | PDR |
| Max dose for infants | 0.2 | ml/kg/dose | 0.2 | ml/kg/dose | intravenous injection, IV | Dotarem Injection | gadoterate meglumine | PDR |
| Max dose for infants | 0.1 | mmoL/kg/dose | 0.1 | mmoL/kg/dose | intravenous injection, IV | Dotarem Injection | gadoterate meglumine | PDR |
| Max dose for children | 0.2 | ml/kg/dose | 0.2 | ml/kg/dose | intravenous injection, IV | Dotarem Injection | gadoterate meglumine | PDR |
| Max dose for children | 0.1 | mmol/kg/dose | 0.1 | mmol/kg/dose | intravenous injection, IV | Dotarem Injection | gadoterate meglumine | PDR |
| Max dose for adolescents | 0.2 | ml/kg/dose | 0.2 | mL/kg/dose | intravenous injection, IV | Dotarem Injection | gadoterate meglumine | PDR |
| Max dose for adolescents | 0.1 | mmol/kg/dose | 0.1 | mmol/kg/dose | intravenous injection, IV | Dotarem Injection | gadoterate meglumine | PDR |
| Max dose for adults | 0.2 | ml/kg/dose | 0.2 | ml/kg/dose | intravenous injection, IV | Dotarem Injection | gadoterate meglumine | PDR |
| Max dose for adults | 0.1 | mmoL/kg/dose | 0.1 | mmoL/kg/dose | intravenous injection, IV | Dotarem Injection | gadoterate meglumine | PDR |
| Max dose for geriatric | 0.2 | ml/kg/dose | 0.2 | ml/kg/dose | intravenous injection, IV | Dotarem Injection | gadoterate meglumine | PDR |
| Max dose for geriatric | 0.1 | mmol/kg/dose | 0.1 | mmol/kg/dose | intravenous injection, IV | Dotarem Injection | gadoterate meglumine | PDR |