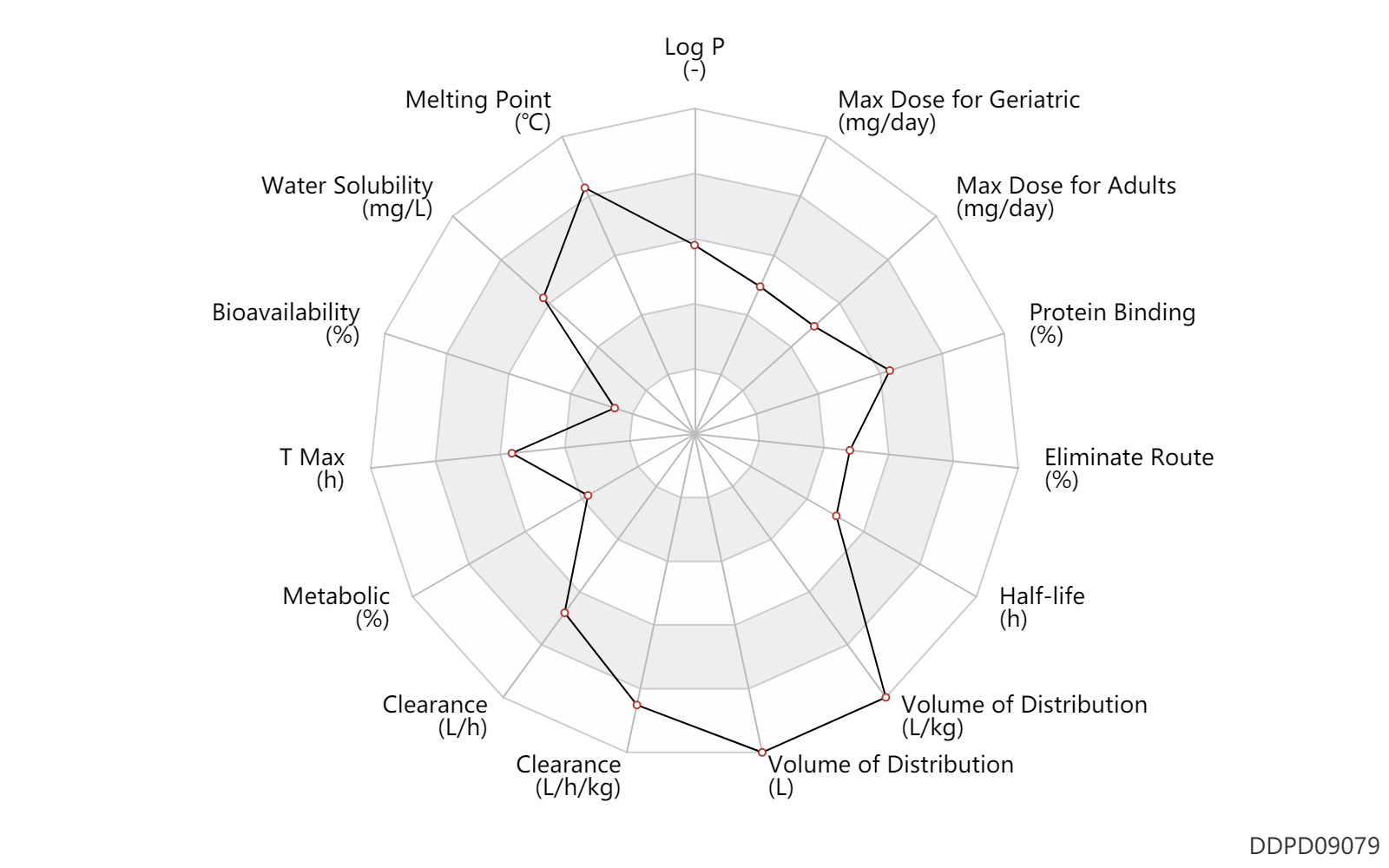
| Property Name |
Property Value |
Unit |
Raw Value |
Raw Unit |
Annotation |
Reference |
| Bioavailability |
4.7 |
% |
4.7 |
% |
PO, oral; patients; fasting; |
DRUGBANK |
| T Max |
2.0 |
h |
2 |
h |
PO, oral; patients; fasting; |
DRUGBANK |
T Max |
4.0 |
h |
4 |
h |
PO, oral; 421AA genotype; food; |
DRUGBANK |
| Metabolic |
5.0 |
% |
5 |
% |
|
DRUGBANK |
Metabolic |
0.0300 |
% |
0.03 |
% |
Urinary excretion; |
DRUGBANK |
Metabolic |
0.0100 |
% |
0.01 |
% |
Urinary excretion; |
DRUGBANK |
| Clearance |
83.4 |
L/h |
1390.0 |
ml/min |
Plasma clearance; |
DRUGBANK |
Clearance |
1.2 |
L/h |
20.0 |
ml/min |
Renal clearance; |
DRUGBANK |
Clearance |
1.2 |
L/h/kg |
19.9 |
ml/min/kg |
intravenous injection, IV; human, homo sapiens; |
Human Intravenous Pharmacokinetic Dataset |
| Volume of Distribution |
1050.0 |
L |
1050.0 |
L |
intravenous injection, IV; |
DRUGBANK |
Volume of Distribution |
15.0 |
L/kg |
15 |
L/kg |
intravenous injection, IV; human, homo sapiens; |
Human Intravenous Pharmacokinetic Dataset |
| Half-life |
12.5 |
h |
~10-15 |
h |
elimination half-life; |
DRUGBANK |
Half-life |
9.5 |
h |
~9.5 |
h |
effective half-life; idiopathic pulmonary fibrosis; |
DRUGBANK |
Half-life |
9.5 |
h |
9.5 |
h |
intravenous injection, IV; human, homo sapiens; |
Human Intravenous Pharmacokinetic Dataset |
| Eliminate Route |
93.4 |
% |
93.4 |
% |
Faeces excretion; |
DRUGBANK |
Eliminate Route |
0.0500 |
% |
0.05 |
% |
PO, oral; Unchanged drug; |
DRUGBANK |
Eliminate Route |
1.4 |
% |
1.4 |
% |
intravenous injection, IV; Unchanged drug; |
DRUGBANK |
| Protein Binding |
97.8 |
% |
97.8 |
% |
plasma proteins; |
DRUGBANK |