Basic Information
| Drug ID | DDPD09073 |

|
| Drug Name | Palbociclib | |
| Molecular Weight | 447.5328 | |
| Molecular Formula | C24H29N7O2 | |
| CAS Number | 571190-30-2 | |
| SMILES | CC(=O)C1=C(C)C2=CN=C(NC3=NC=C(C=C3)N3CCNCC3)N=C2N(C2CCCC2)C1=O | |
| External Links | ||
| DRUGBANK | DB09073 | |
| PubChem Compound | 5330286 | |
| PDR | 3679 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
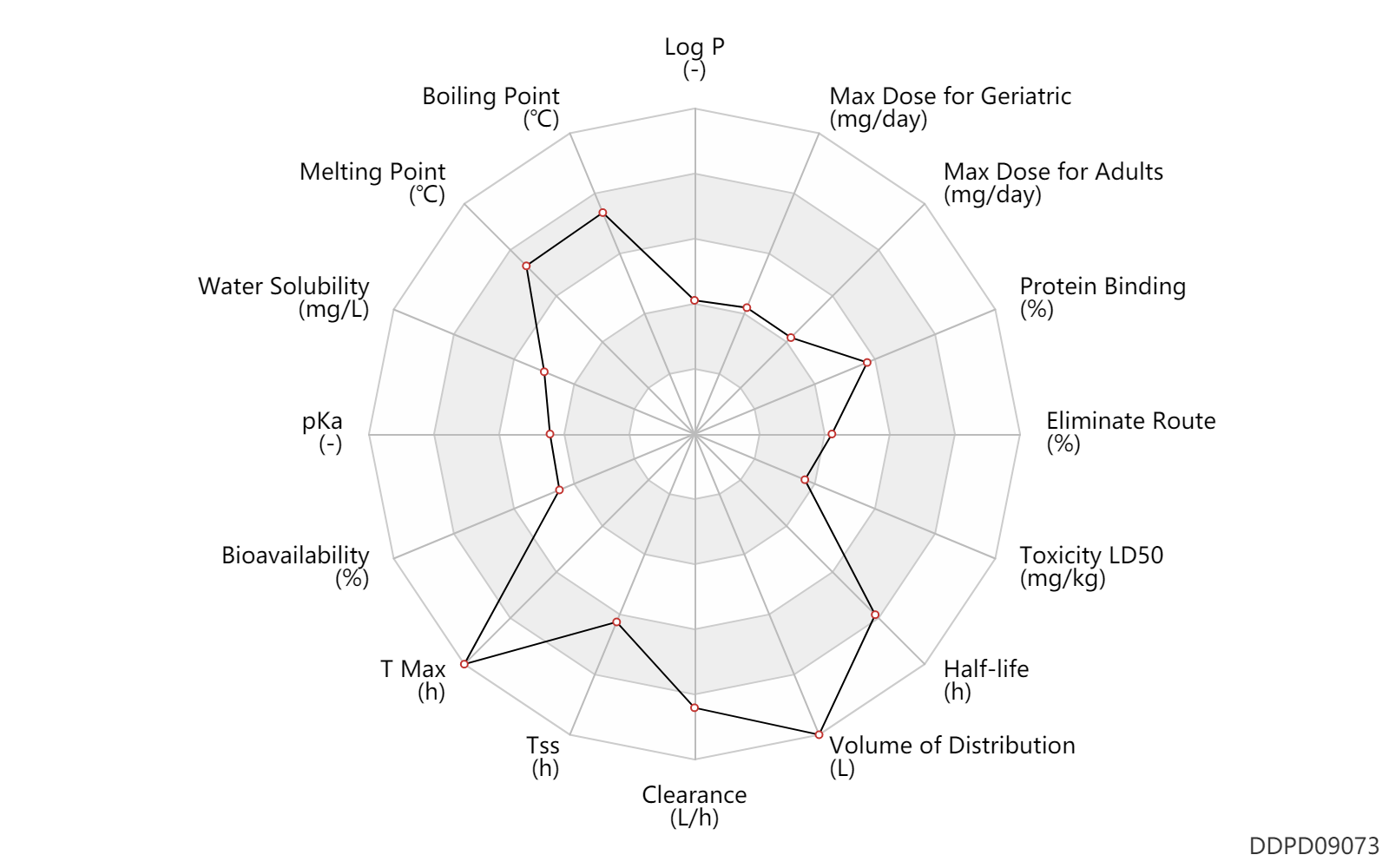
| Log P | 0.99 | - | 0.99 | - | IBRANCE (palbociclib) monograph |
| Boiling Point | 711.5 | ℃ | 711.5 | ℃ | 'MSDS' |
| Melting Point | 264.5 | ℃ | 263-266 | ℃ | 'MSDS' |
| Water Solubility | 10000.0 | mg/L | 10 | mg/ml | 'MSDS' |
| pKa | 7.4 | - | 7.4,3.9 | - | DRUGBANK | pKa | 3.9 | - | 7.4,3.9 | - | 'FDA Label' |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
| Bioavailability | 46.0 | % | 46 | % | PO, oral; | DRUGBANK |
| T Max | 9.0 | h | 6-12 | h | PO, oral; | DRUGBANK |
| Tss | 192.0 | h | 8 | day | PO, oral; | DRUGBANK |
| Clearance | 63.1 | L/h | 63.1 | L/h | apparent clearance; PO, oral; | DRUGBANK |
| Volume of Distribution | 2583.0 | L | 2583.0 | L | Apparent volume of distribution; | DRUGBANK |
| Half-life | 29.0 | h | 29 | h | elimination half-life; | DRUGBANK |
| Toxicity LD50 | 100.0 | mg/kg | 100.0 | mg/kg | PO, oral; | DRUGBANK |
| Eliminate Route | 17.5 | % | 17.5 | % | Urinary excretion; | DRUGBANK |
| Protein Binding | 85.0 | % | 85 | % | plasma proteins; high protein binding; human, homo sapiens; | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for adults | 125.0 | mg/day | 125 | mg/day | PO, oral | Ibrance | palbociclib | PDR |
| Max dose for geriatric | 125.0 | mg/day | 125 | mg/day | PO, oral | Ibrance | palbociclib | PDR |