Basic Information
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
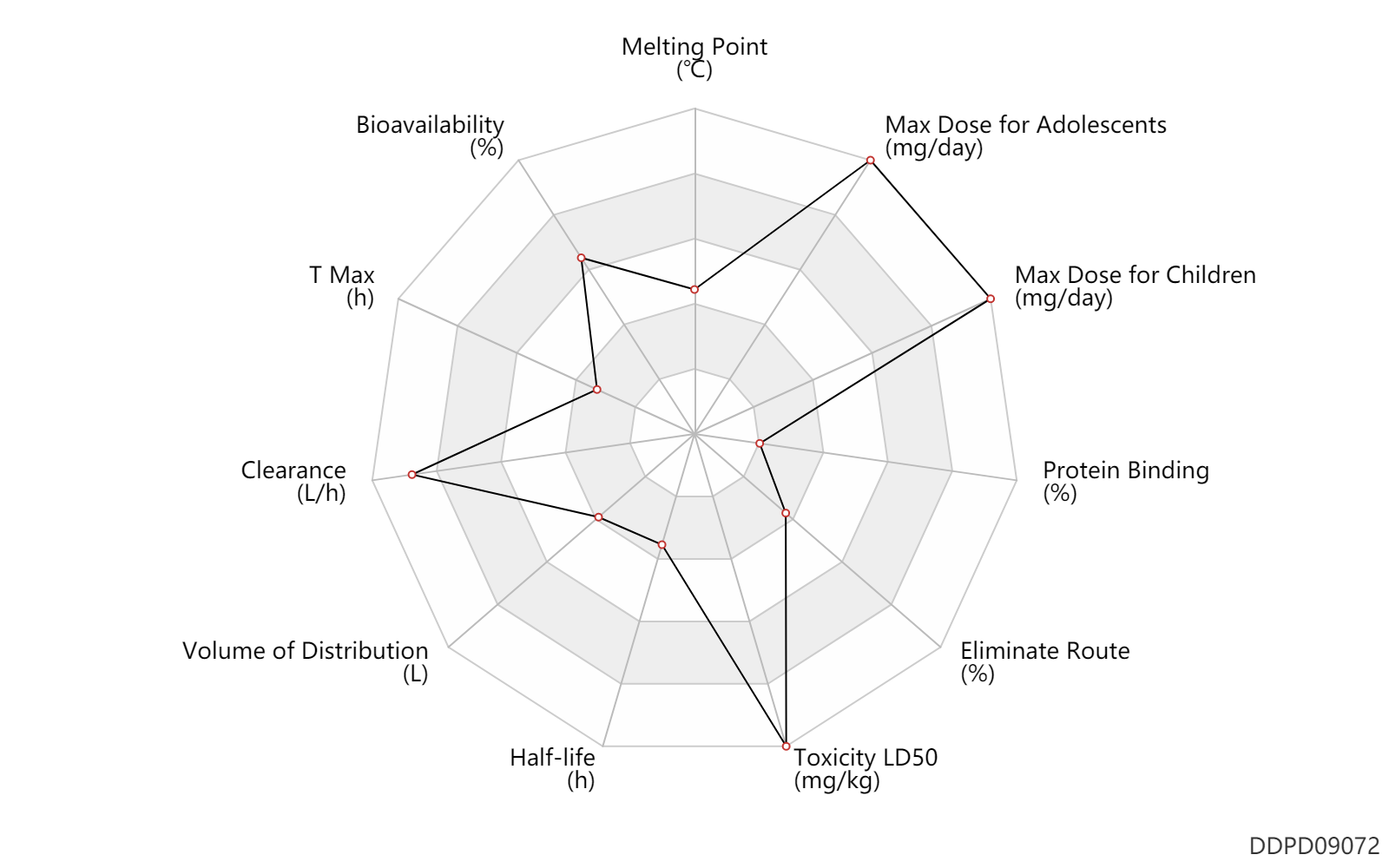
| Melting Point | 145.5 | ℃ | 145-146 | ℃ | MSDS |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
| Bioavailability | 88.0 | % | 88 | % | DRUGBANK | |
| T Max | 0.69 | h | 30.7-51.9 | min | DRUGBANK | |
| Clearance | 67.7 | L/h | 895-1361 | ml/min | Total clearance; | DRUGBANK |
| Volume of Distribution | 52.7 | L | 37.7-67.7 | L | DRUGBANK | |
| Half-life | 0.75 | h | 0.5-1 | h | DRUGBANK | |
| Toxicity LD50 | 9690.0 | mg/kg | 9690.0 | mg/kg | PO, oral; Rattus, Rat; | DRUGBANK |
| Eliminate Route | 5.0 | % | <5 | % | Urinary excretion; Unchanged drug; | DRUGBANK |
| Protein Binding | 1.0 | % | <1 | % | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for children | 9000.0 | mg/day | 9 | g/day | PO, oral | Xyrem | sodium oxybate | PDR |
| Max dose for children | 7500.0 | mg/day | 7.5 | g/day | PO, oral | Xyrem | sodium oxybate | PDR |
| Max dose for children | 6000.0 | mg/day | 6 | g/day | PO, oral | Xyrem | sodium oxybate | PDR |
| Max dose for children | 6000.0 | mg/day | 6 | g/day | PO, oral | Xyrem | sodium oxybate | PDR |
| Max dose for adolescents | 9000.0 | mg/day | 9 | g/day | PO, oral | Xyrem | sodium oxybate | PDR |
| Max dose for adolescents | 7500.0 | mg/day | 7.5 | g/day | PO, oral | Xyrem | sodium oxybate | PDR |
| Max dose for adolescents | 6000.0 | mg/day | 6 | g/day | PO, oral | Xyrem | sodium oxybate | PDR |
| Max dose for adults | 9.0 | g/night | 9 | g/night | PO, oral | Xyrem | sodium oxybate | PDR |
| Max dose for geriatric | 9.0 | g/night | 9 | g/night | PO, oral | Xyrem | sodium oxybate | PDR |