Basic Information
| Drug ID | DDPD09065 |

|
| Drug Name | Cobicistat | |
| Molecular Weight | 776.03 | |
| Molecular Formula | C40H53N7O5S2 | |
| CAS Number | 1004316-88-4 | |
| SMILES | CC(C)C1=NC(CN(C)C(=O)N[C@@H](CCN2CCOCC2)C(=O)N[C@H](CC[C@H](CC2=CC=CC=C2)NC(=O)OCC2=CN=CS2)CC2=CC=CC=C2)=CS1 | |
| External Links | ||
| DRUGBANK | DB09065 | |
| PubChem Compound | 25151504 | |
| PDR | 3616 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
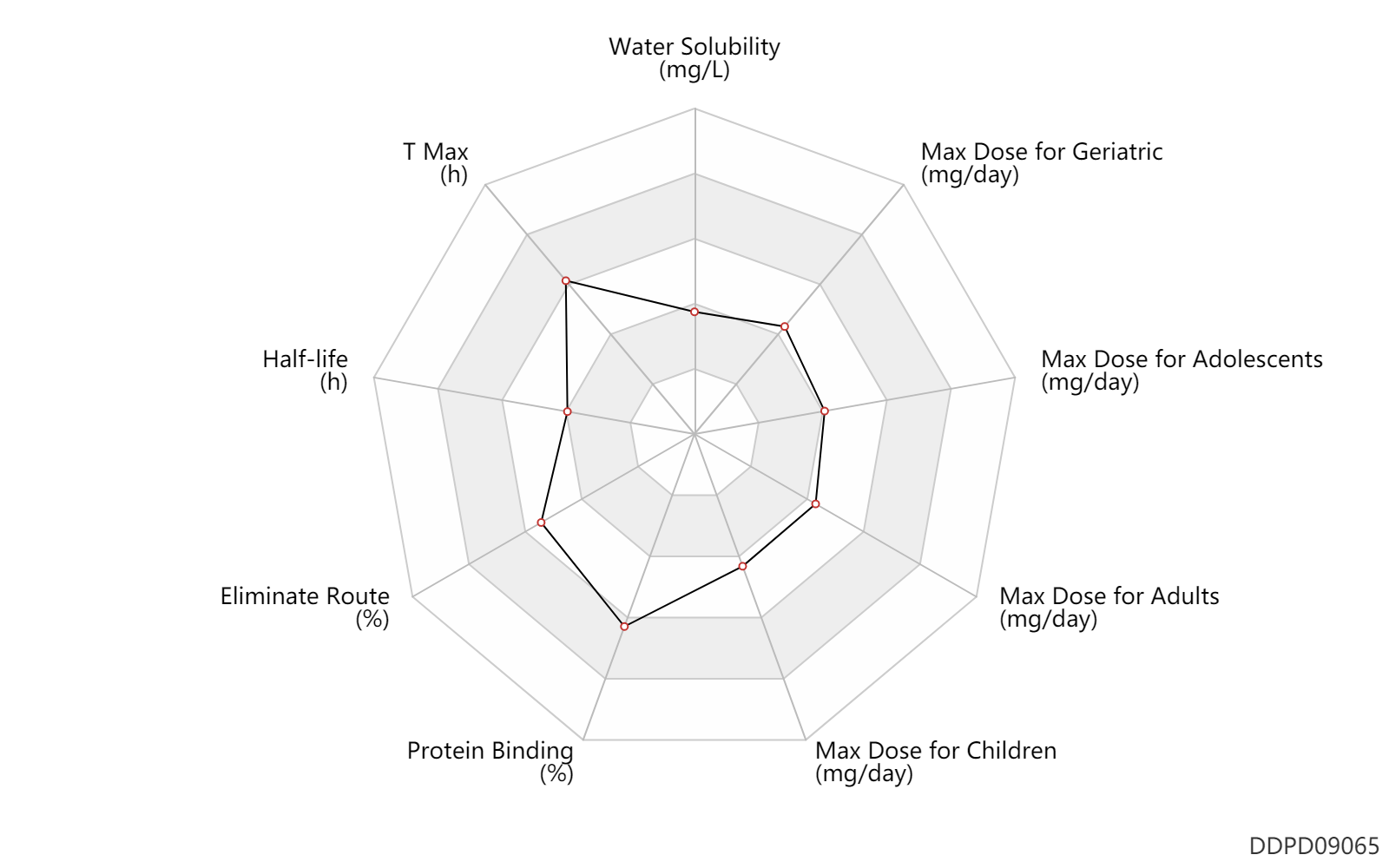
| Water Solubility | 100.0 | mg/L | 0.1 | mg/ml | FDA Label |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
| T Max | 3.5 | h | 3.5 | h | DRUGBANK | |
| Half-life | 3.5 | h | ~3-4 | h | terminal half-life; | DRUGBANK |
| Eliminate Route | 86.2 | % | 86.2 | % | Faeces excretion; Drug combination; | DRUGBANK | Eliminate Route | 8.2 | % | 8.2 | % | Urinary excretion; Drug combination; | DRUGBANK |
| Protein Binding | 97.5 | % | 97-98 | % | plasma proteins; human, homo sapiens; | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for children | 150.0 | mg/day | 150 | mg/day | PO, oral | Tybost | cobicistat | PDR |
| Max dose for adolescents | 150.0 | mg/day | 150 | mg/day | PO, oral | Tybost | cobicistat | PDR |
| Max dose for adults | 150.0 | mg/day | 150 | mg/day | PO, oral | Tybost | cobicistat | PDR |
| Max dose for geriatric | 150.0 | mg/day | 150 | mg/day | PO, oral | Tybost | cobicistat | PDR |