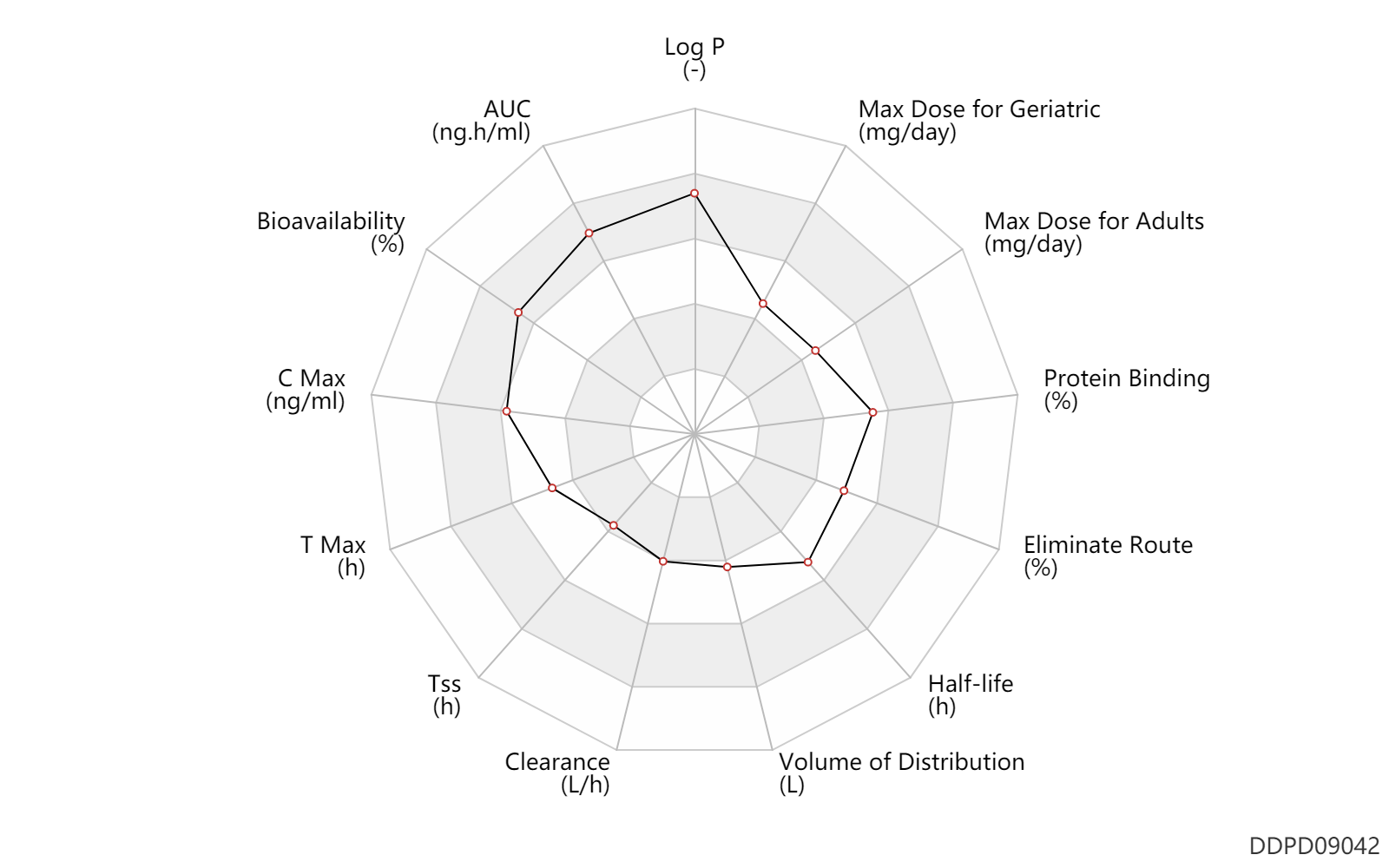
| Property Name |
Property Value |
Unit |
Raw Value |
Raw Unit |
Annotation |
Reference |
| AUC |
25600.0 |
ng.h/ml |
25.6±8.4 |
mcg.h/ml |
PO, oral; |
DRUGBANK |
AUC |
29200.0 |
ng.h/ml |
29.2±6.2 |
mcg.h/ml |
intravenous injection, IV; |
DRUGBANK |
AUC |
23800.0 |
ng.h/ml |
23.8±6.8 |
mcg.h/ml |
Oral single dose; |
DRUGBANK |
AUC |
26600.0 |
ng.h/ml |
26.6±5.2 |
mcg.h/ml |
|
DRUGBANK |
| Bioavailability |
91.0 |
% |
91 |
% |
PO, oral; |
DRUGBANK |
| C Max |
2000.0 |
ng/ml |
2.0±0.7 |
mcg/ml |
Oral single dose; |
DRUGBANK |
C Max |
2300.0 |
ng/ml |
2.3±0.6 |
mcg/ml |
|
DRUGBANK |
| Css |
2200.0 |
ng/ml |
2.2±0.6 |
mcg/ml |
PO, oral; |
DRUGBANK |
Css |
3000.0 |
ng/ml |
3.0±0.7 |
mcg/ml |
intravenous injection, IV; |
DRUGBANK |
| T Max |
3.0 |
h |
3 |
h |
PO, oral; |
DRUGBANK |
T Max |
1.0 |
h |
1 |
h |
intravenous injection, IV; |
DRUGBANK |
T Max |
3.5 |
h |
3.5(1.0-6.0) |
h |
PO, oral; |
DRUGBANK |
T Max |
1.2 |
h |
1.2(0.9-1.5) |
h |
intravenous injection, IV; |
DRUGBANK |
T Max |
2.5 |
h |
2.5(1.0-8.0) |
h |
Oral single dose; |
DRUGBANK |
T Max |
1.1 |
h |
1.1(0.9-1.5) |
h |
|
DRUGBANK |
| Tss |
72.0 |
h |
3 |
day |
PO, oral; |
DRUGBANK |
Tss |
72.0 |
h |
3 |
day |
intravenous injection, IV; |
DRUGBANK |
| Clearance |
6.9 |
L/h |
6.9±1.7 |
L/h |
apparent clearance; Oral single dose; |
DRUGBANK |
Clearance |
8.4 |
L/h |
8.4±2.1 |
L/h |
apparent clearance; at steady state; |
DRUGBANK |
Clearance |
6.4 |
L/h |
6.4±1.2 |
L/h |
Total clearance; Single dose; |
DRUGBANK |
Clearance |
5.9 |
L/h |
5.9±1.4 |
L/h |
Total clearance; at steady state; |
DRUGBANK |
| Volume of Distribution |
73.5 |
L |
67-80 |
L |
intravenous injection, IV; Single dose; |
DRUGBANK |
Volume of Distribution |
108.0 |
L |
108 ± 21 |
L |
PO, oral; |
DRUGBANK |
Volume of Distribution |
113.3 |
L |
113.3 ± 19.3 |
L |
Apparent volume of distribution; Oral single dose; |
DRUGBANK |
| Half-life |
12.0 |
h |
~12 |
h |
|
DRUGBANK |
| Eliminate Route |
82.0 |
% |
~82 |
% |
Faeces excretion; Oral single dose; |
DRUGBANK |
Eliminate Route |
18.0 |
% |
~18 |
% |
Urinary excretion; Oral single dose; |
DRUGBANK |
Eliminate Route |
3.0 |
% |
3 |
% |
Oral single dose; Unchanged drug; |
DRUGBANK |
| Protein Binding |
80.0 |
% |
~70-90 |
% |
plasma proteins; human, homo sapiens; |
DRUGBANK |