Basic Information
| Drug ID | DDPD09031 |

|
| Drug Name | Miltefosine | |
| Molecular Weight | 407.576 | |
| Molecular Formula | C21H46NO4P | |
| CAS Number | 58066-85-6 | |
| SMILES | CCCCCCCCCCCCCCCCOP([O-])(=O)OCC[N+](C)(C)C | |
| External Links | ||
| DRUGBANK | DB09031 | |
| PubChem Compound | 3599 | |
| PDR | 3607 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
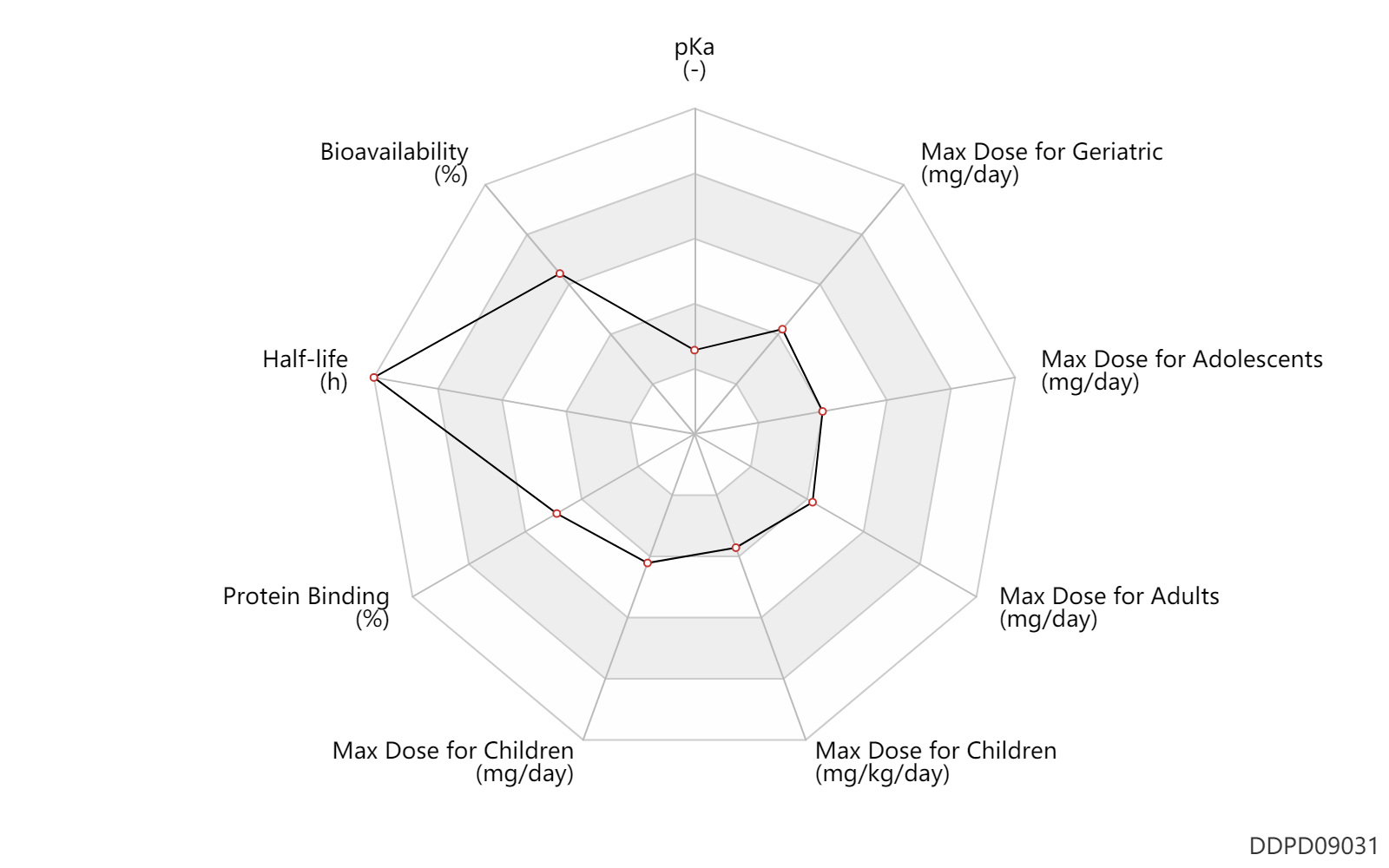
| pKa | 2.0 | - | ~2 | - | DRUGBANK |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
| Bioavailability | 82.0 | % | 82 | % | PO, oral; Rattus, Rat; | DRUGBANK | Bioavailability | 94.0 | % | 94 | % | PO, oral; dog; | DRUGBANK |
| Half-life | 169.2 | h | 7.05(5.45-9.10) | day | elimination half-life; | DRUGBANK | Half-life | 741.6 | h | 30.9(30.8-31.2) | day | terminal half-life; | DRUGBANK |
| Protein Binding | 97.0 | % | 96-98 | % | plasma proteins; | DRUGBANK | Protein Binding | 97.0 | % | 97 | % | DRUGBANK | Protein Binding | 3.0 | % | 3 | % | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for children | 150.0 | mg/day | 150 | mg/day | PO, oral | Impavido | miltefosine | PDR |
| Max dose for children | 100.0 | mg/day | 100 | mg/day | PO, oral | Impavido | miltefosine | PDR |
| Max dose for children | 2.5 | mg/kg/day | 2.5 | mg/kg/day | PO, oral | Impavido | miltefosine | PDR |
| Max dose for children | 2.5 | mg/kg/day | 2.5 | mg/kg/day | PO, oral | Impavido | miltefosine | PDR |
| Max dose for adolescents | 150.0 | mg/day | 150 | mg/day | PO, oral | Impavido | miltefosine | PDR |
| Max dose for adolescents | 100.0 | mg/day | 100 | mg/day | PO, oral | Impavido | miltefosine | PDR |
| Max dose for adults | 150.0 | mg/day | 150 | mg/day | PO, oral | Impavido | miltefosine | PDR |
| Max dose for adults | 100.0 | mg/day | 100 | mg/day | PO, oral | Impavido | miltefosine | PDR |
| Max dose for geriatric | 150.0 | mg/day | 150 | mg/day | PO, oral | Impavido | miltefosine | PDR |
| Max dose for geriatric | 100.0 | mg/day | 100 | mg/day | PO, oral | Impavido | miltefosine | PDR |