Basic Information
| Drug ID | DDPD08930 |

|
| Drug Name | Dolutegravir | |
| Molecular Weight | 419.3788 | |
| Molecular Formula | C20H19F2N3O5 | |
| CAS Number | 1051375-16-6 | |
| SMILES | [H][C@]12CN3C=C(C(=O)NCC4=CC=C(F)C=C4F)C(=O)C(O)=C3C(=O)N1[C@H](C)CCO2 | |
| External Links | ||
| DRUGBANK | DB08930 | |
| PubChem Compound | 54726191 | |
| PDR | 3246 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
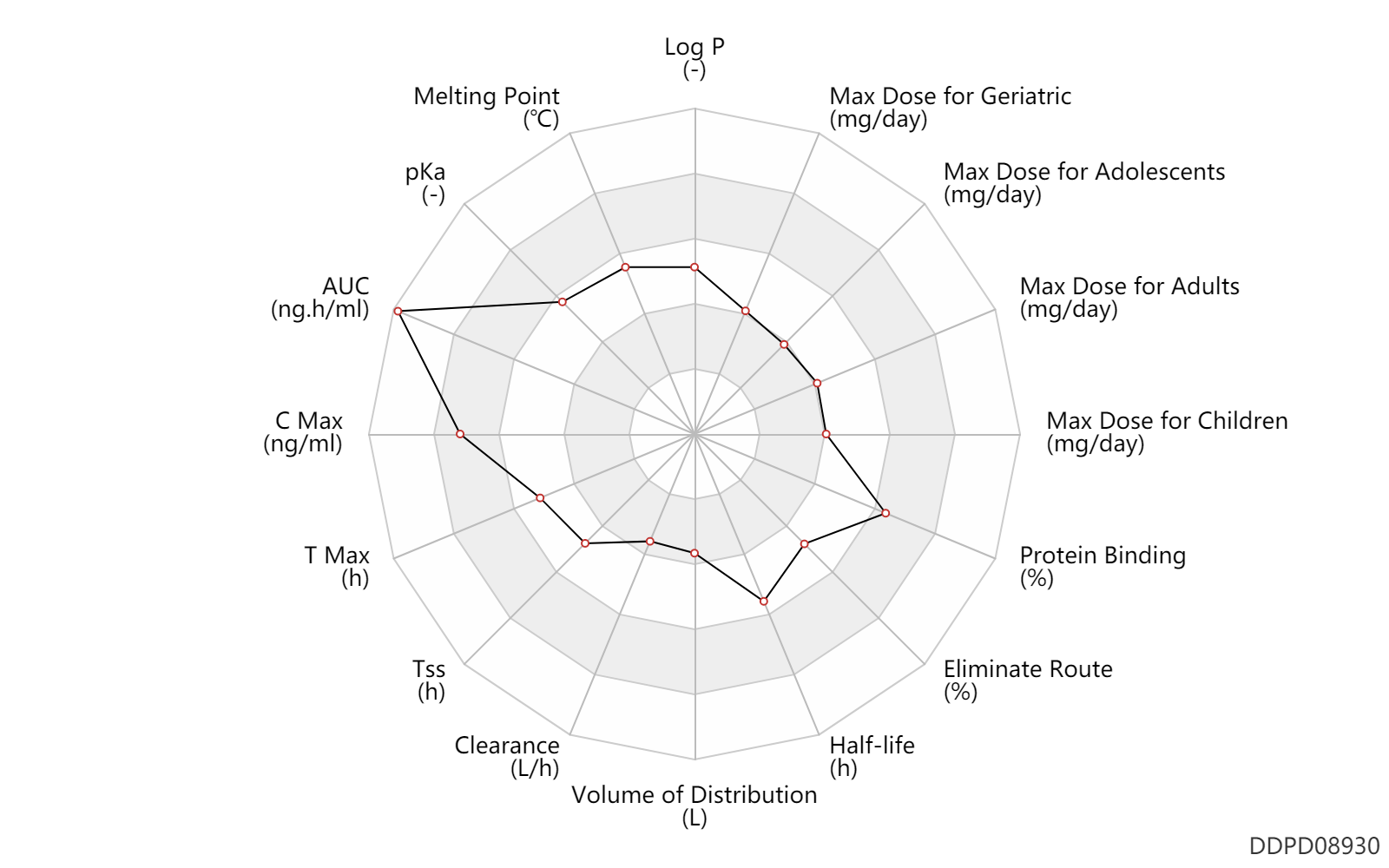
| Log P | 2.2 | - | 2.2 | - | MSDS |
| Melting Point | 191.5 | ℃ | 190-193 | ℃ | MSDS |
| pKa | 8.2 | - | 8.2 | - | MSDS |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
| AUC | 53600.0 | ng.h/ml | 53.6 | mcg.h/ml | PO, oral; AIDS,HIV; | DRUGBANK | AUC | 46000.0 | ng.h/ml | 46.0 | mcg.h/ml | PO, oral; Caucasian; AIDS,HIV; | DRUGBANK |
| C Max | 3670.0 | ng/ml | 3.67 | mcg/ml | PO, oral; AIDS,HIV; | DRUGBANK | C Max | 3490.0 | ng/ml | 3.49 | mcg/ml | PO, oral; Caucasian; AIDS,HIV; | DRUGBANK |
| T Max | 2.5 | h | 2-3 | h | PO, oral; AIDS,HIV; | DRUGBANK |
| Tss | 120.0 | h | 5 | day | PO, oral; AIDS,HIV; | DRUGBANK |
| Clearance | 1.0 | L/h | 1.0 | L/h | apparent clearance; | DRUGBANK |
| Volume of Distribution | 17.4 | L | 17.4 | L | Apparent volume of distribution; | DRUGBANK |
| Half-life | 14.0 | h | 14 | h | DRUGBANK | |
| Eliminate Route | 31.0 | % | 31 | % | Urinary excretion; | DRUGBANK |
| Protein Binding | 98.9 | % | 98.9 | % | plasma proteins; human, homo sapiens; | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for children | 100.0 | mg/day | 100 | mg/day | PO, oral | Tivicay | dolutegravir | PDR |
| Max dose for children | 70.0 | mg/day | 70 | mg/day | PO, oral | Tivicay | dolutegravir | PDR |
| Max dose for adolescents | 100.0 | mg/day | 100 | mg/day | PO, oral | Tivicay | dolutegravir | PDR |
| Max dose for adolescents | 70.0 | mg/day | 70 | mg/day | PO, oral | Tivicay | dolutegravir | PDR |
| Max dose for adults | 100.0 | mg/day | 100 | mg/day | PO, oral | Tivicay | dolutegravir | PDR |
| Max dose for geriatric | 100.0 | mg/day | 100 | mg/day | PO, oral | Tivicay | dolutegravir | PDR |