Basic Information
Experimental Physicochemical Property
Not Available
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
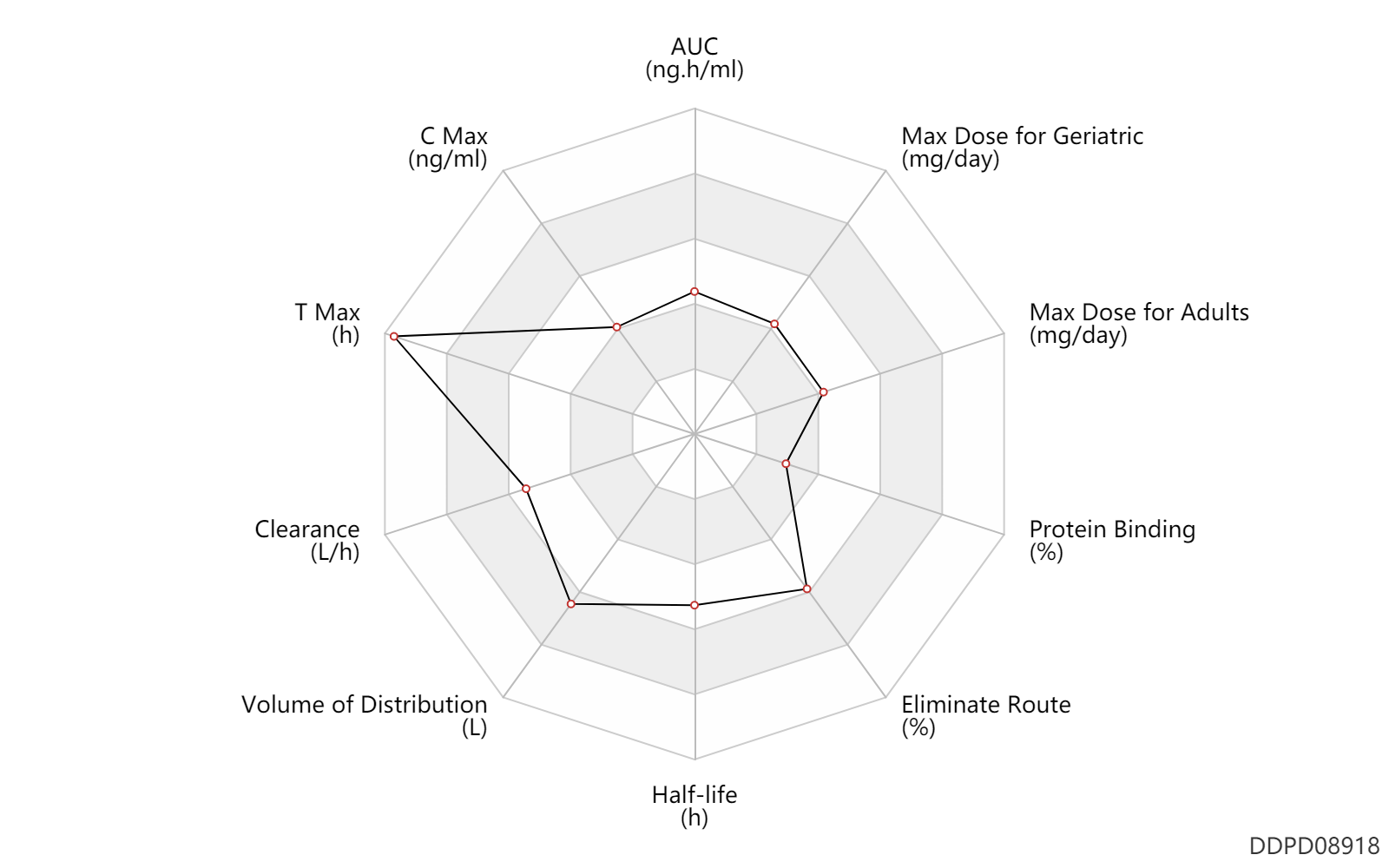
| AUC | 5196.0 | ng.h/ml | 5196.0 | ng.h/ml | Capsule, PO, Oral; extended release formulation; | DRUGBANK |
| C Max | 341.0 | ng/ml | 341 | ng/ml | Capsule, PO, Oral; extended release formulation; | DRUGBANK |
| T Max | 7.0 | h | 6-8 | h | PO, oral; | DRUGBANK |
| Clearance | 25.0 | L/h | 21-29 | L/h | apparent clearance; | DRUGBANK |
| Volume of Distribution | 430.0 | L | 387-473 | L | Apparent volume of distribution; | DRUGBANK |
| Half-life | 12.0 | h | 12 | h | DRUGBANK | |
| Eliminate Route | 58.0 | % | 58 | % | Urinary excretion; Unchanged drug; | DRUGBANK |
| Protein Binding | 22.0 | % | 22 | % | plasma proteins; human, homo sapiens; | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for adults | 120.0 | mg/day | 120 | mg/day | PO, oral | Fetzima | levomilnacipran | PDR |
| Max dose for geriatric | 120.0 | mg/day | 120 | mg/day | PO, oral | Fetzima | levomilnacipran | PDR |