Basic Information
| Drug ID | DDPD08899 |

|
| Drug Name | Enzalutamide | |
| Molecular Weight | 464.436 | |
| Molecular Formula | C21H16F4N4O2S | |
| CAS Number | 915087-33-1 | |
| SMILES | CNC(=O)C1=C(F)C=C(C=C1)N1C(=S)N(C(=O)C1(C)C)C1=CC=C(C#N)C(=C1)C(F)(F)F | |
| External Links | ||
| DRUGBANK | DB08899 | |
| PubChem Compound | 15951529 | |
| PDR | 2590 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
Not Available
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
| Css | 16600.0 | ng/ml | 16.6 | ug/ml | DRUGBANK | Css | 12700.0 | ng/ml | 12.7 | ug/ml | Active metabolite; | DRUGBANK |
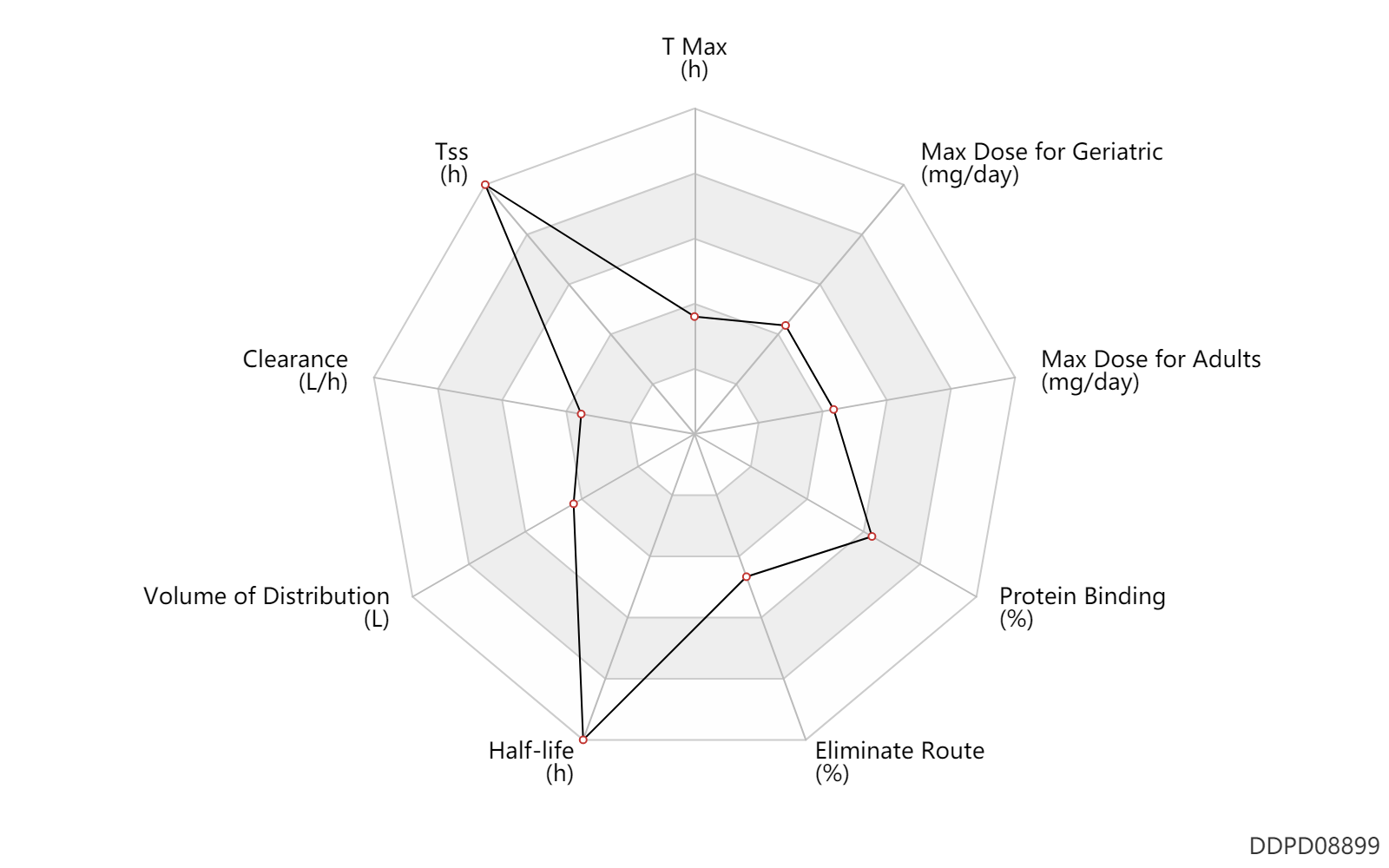
| T Max | 1.0 | h | 1 | h | DRUGBANK | |
| Tss | 672.0 | h | 28 | day | Active metabolite; | DRUGBANK |
| Clearance | 0.56 | L/h | 0.56 | L/h | apparent clearance; Oral single dose; | DRUGBANK |
| Volume of Distribution | 110.0 | L | 110.0 | L | Apparent volume of distribution; Oral single dose; | DRUGBANK |
| Half-life | 139.2 | h | 5.8(2.8-10.2) | day | terminal half-life; patients; Oral single dose; | DRUGBANK | Half-life | 196.8 | h | 7.8-8.6 | day | terminal half-life; normal,healthy; Oral single dose; | DRUGBANK |
| Eliminate Route | 71.0 | % | 71 | % | Urinary excretion; | DRUGBANK | Eliminate Route | 14.0 | % | 14 | % | Faeces excretion; | DRUGBANK | Eliminate Route | 0.0560 | % | 0.056 | % | Faeces excretion; Unchanged drug; | DRUGBANK |
| Protein Binding | 97.5 | % | 97-98 | % | plasma proteins; | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for adults | 160.0 | mg/day | 160 | mg/day | PO, oral | Xtandi | enzalutamide | PDR |
| Max dose for geriatric | 160.0 | mg/day | 160 | mg/day | PO, oral | Xtandi | enzalutamide | PDR |