Basic Information
| Drug ID | DDPD08881 |

|
| Drug Name | Vemurafenib | |
| Molecular Weight | 489.922 | |
| Molecular Formula | C23H18ClF2N3O3S | |
| CAS Number | 918504-65-1 | |
| SMILES | CCCS(=O)(=O)NC1=C(F)C(C(=O)C2=CNC3=NC=C(C=C23)C2=CC=C(Cl)C=C2)=C(F)C=C1 | |
| External Links | ||
| DRUGBANK | DB08881 | |
| PubChem Compound | 42611257 | |
| PDR | 1473 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
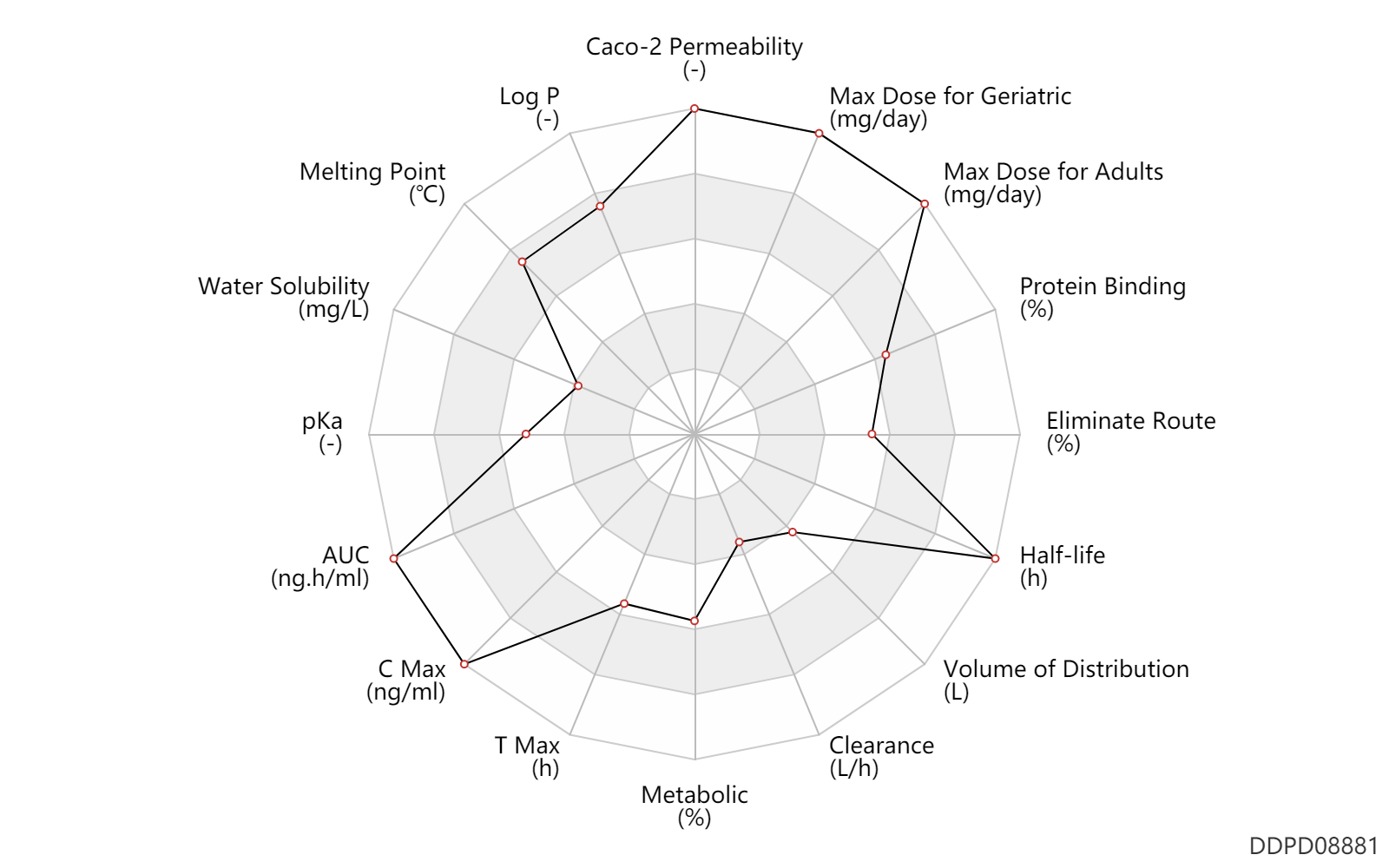
| Caco-2 Permeability | 2.9e-06 | - | 2.9e-06 | - | FDA review |
| Log P | 5.1 | - | 5.1 | - | MSDS |
| Melting Point | 272.0 | ℃ | 272 | ℃ | MSDS |
| Water Solubility | 1000.0 | mg/L | <1 | mg/ml | MSDS |
| pKa | 7.1 | - | 7.1 | - | Royal Soc Chem |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
| AUC | 601000.0 | ng.h/ml | 601.0 | mcg.h/ml | PO, oral; patients; | DRUGBANK |
| C Max | 62000.0 | ng/ml | 62 | mcg/ml | PO, oral; patients; | DRUGBANK |
| T Max | 3.0 | h | 3 | h | PO, oral; patients; | DRUGBANK |
| Metabolic | 5.0 | % | 5 | % | DRUGBANK | Metabolic | 95.0 | % | 95 | % | Unchanged drug; | DRUGBANK |
| Clearance | 1.3 | L/h | 31.0 | L/day | Total clearance; | DRUGBANK |
| Volume of Distribution | 106.0 | L | 106.0 | L | DRUGBANK | |
| Half-life | 57.0 | h | 57(30-120) | h | elimination half-life; | DRUGBANK |
| Eliminate Route | 94.0 | % | 94 | % | Faeces excretion; | DRUGBANK | Eliminate Route | 1.0 | % | 1 | % | Urinary excretion; | DRUGBANK |
| Protein Binding | 99.0 | % | >99 | % | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for adults | 1920.0 | mg/day | 1920 | mg/day | PO, oral | Zelboraf | vemurafenib | PDR |
| Max dose for geriatric | 1920.0 | mg/day | 1920 | mg/day | PO, oral | Zelboraf | vemurafenib | PDR |