Basic Information
| Drug ID | DDPD08877 |

|
| Drug Name | Ruxolitinib | |
| Molecular Weight | 306.365 | |
| Molecular Formula | C17H18N6 | |
| CAS Number | 941678-49-5 | |
| SMILES | N#CC[C@H](C1CCCC1)N1C=C(C=N1)C1=C2C=CNC2=NC=N1 | |
| External Links | ||
| DRUGBANK | DB08877 | |
| PubChem Compound | 25126798 | |
| PDR | 1499 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
Not Available
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
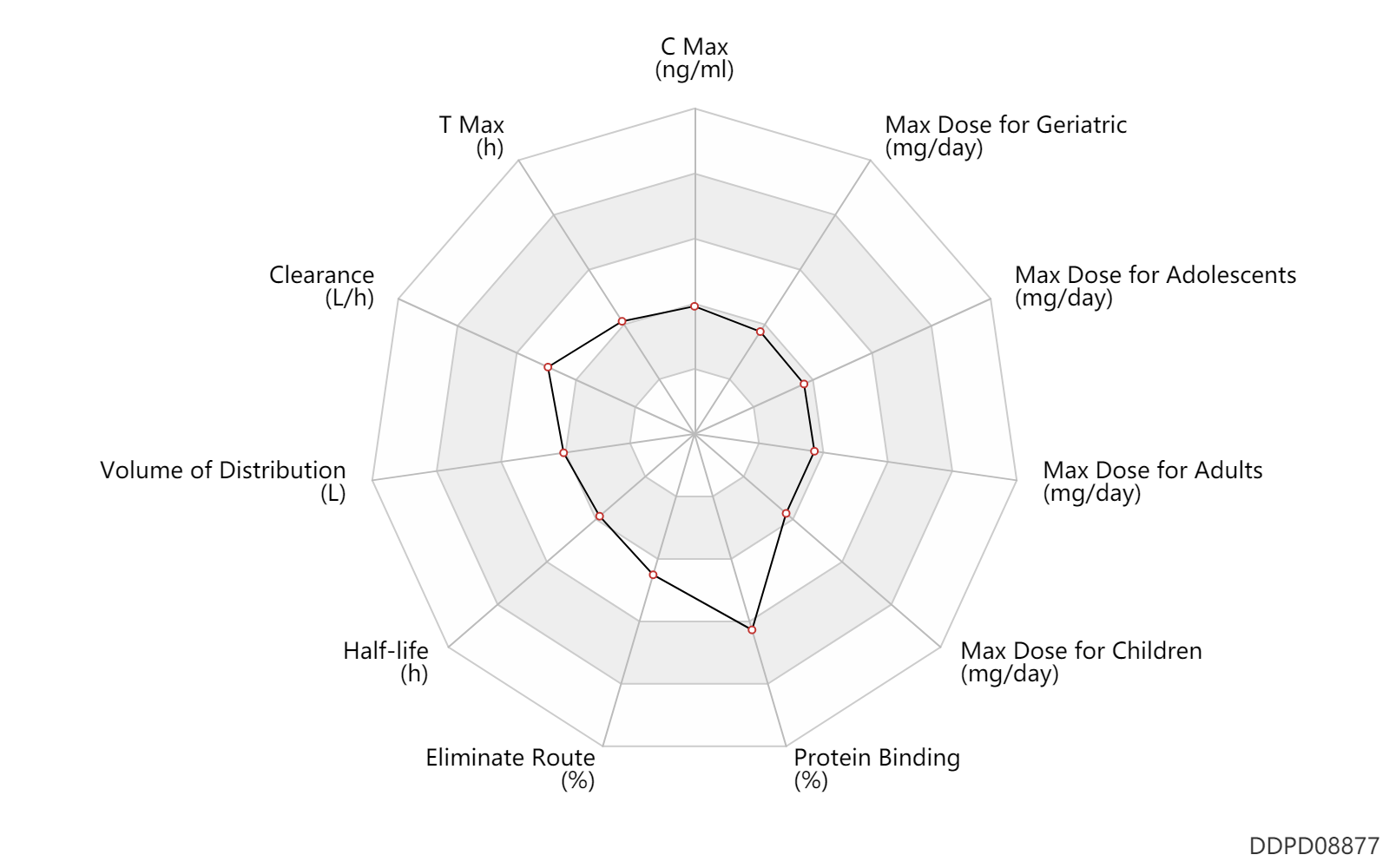
| C Max | 198.8 | ng/ml | 649 | nmol/L | PO, oral; normal,healthy; | DRUGBANK |
| T Max | 1.5 | h | 1.5 | h | PO, oral; normal,healthy; | DRUGBANK |
| Clearance | 18.7 | L/h | 18.7 | L/h | normal,healthy; | DRUGBANK |
| Volume of Distribution | 76.6 | L | 76.6 | L | normal,healthy; | DRUGBANK |
| Half-life | 2.8 | h | 2.8 | h | elimination half-life; normal,healthy; | DRUGBANK |
| Eliminate Route | 74.0 | % | 74 | % | Urinary excretion; | DRUGBANK | Eliminate Route | 22.0 | % | 22 | % | Faeces excretion; | DRUGBANK | Eliminate Route | 1.0 | % | <1 | % | Urinary excretion; Unchanged drug; | DRUGBANK | Eliminate Route | 1.0 | % | <1 | % | Faeces excretion; Unchanged drug; | DRUGBANK |
| Protein Binding | 97.0 | % | 97 | % | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Frequency | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Max dose for children | 10.0 | mg/day | 10 | mg/day | PO, oral | bid | Jakafi | ruxolitinib | PDR |
| Max dose for adolescents | 10.0 | mg/day | 10 | mg/day | PO, oral | bid | Jakafi | ruxolitinib | PDR |
| Max dose for adults | 25.0 | mg/day | 25 | mg/day | PO, oral | bid | Jakafi | ruxolitinib | PDR |
| Max dose for adults | 10.0 | mg/day | 10 | mg/day | PO, oral | bid | Jakafi | ruxolitinib | PDR |
| Max dose for geriatric | 25.0 | mg/day | 25 | mg/day | PO, oral | bid | Jakafi | ruxolitinib | PDR |
| Max dose for geriatric | 10.0 | mg/day | 10 | mg/day | PO, oral | bid | Jakafi | ruxolitinib | PDR |