Basic Information
| Drug ID | DDPD08819 |

|
| Drug Name | Tafluprost | |
| Molecular Weight | 452.5313 | |
| Molecular Formula | C25H34F2O5 | |
| CAS Number | 209860-87-7 | |
| SMILES | CC(C)OC(=O)CCC\C=C/C[C@H]1[C@@H](O)C[C@@H](O)[C@@H]1\C=C\C(F)(F)COC1=CC=CC=C1 | |
| External Links | ||
| DRUGBANK | DB08819 | |
| PubChem Compound | 9868491 | |
| PDR | 2579 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
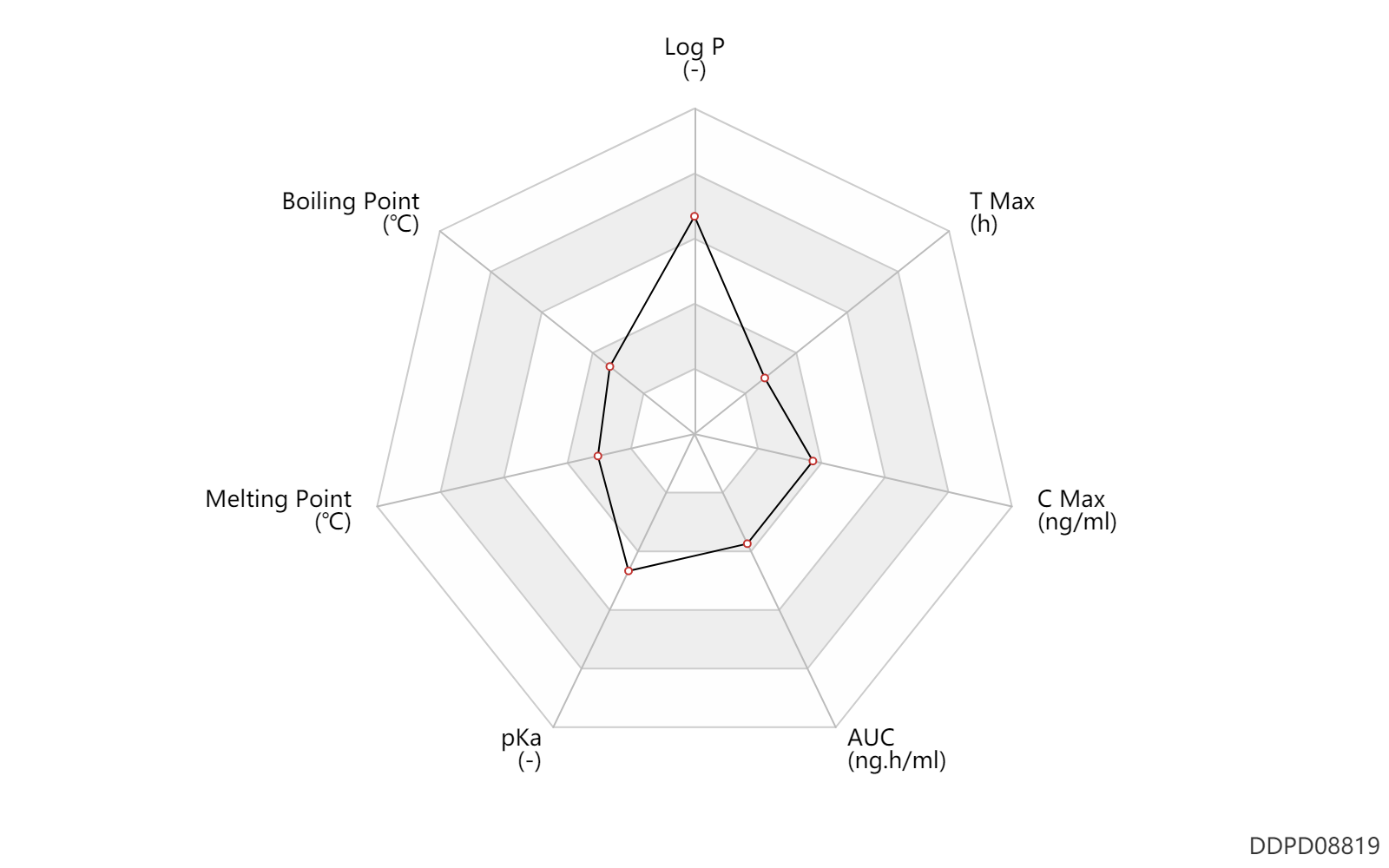
| Log P | 4.05 | - | 4.05 | - | DRUGBANK |
| Boiling Point | 100.0 | ℃ | 100 | ℃ | DRUGBANK |
| Melting Point | 87.5 | ℃ | 87.5 | ℃ | DRUGBANK |
| pKa | 6.1 | - | 5.5-6.7 | - | FDA label |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
| AUC | 0.0688 | ng.h/ml | 394-432 | pg.min/ml | ophthalmic administration; Active metabolite; | DRUGBANK |
| C Max | 0.0260 | ng/ml | 26 | pg/ml | ophthalmic administration; Active metabolite; | DRUGBANK |
| T Max | 0.17 | h | 10 | min | ophthalmic administration; Active metabolite; | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Frequency | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Max dose for adults | 1.0 | drop/day | 1 | drop/day | ophthalmic administration | qd | Zioptan | tafluprost | PDR |
| Max dose for geriatric | 1.0 | drop/day | 1 | drop/day | ophthalmic administration | qd | Zioptan | tafluprost | PDR |