Basic Information
| Drug ID | DDPD06791 |

|
| Drug Name | Lanreotide | |
| Molecular Weight | 1096.33 | |
| Molecular Formula | C54H69N11O10S2 | |
| CAS Number | 108736-35-2 | |
| SMILES | [H]N([H])CCCCC1N([H])C(=O)C(CC2=CN([H])C3=CC=CC=C23)N([H])C(=O)C(CC2=CC=C(O)C=C2)N([H])C(=O)C(CSSCC(N([H])C(=O)C(N([H])C1=O)C(C)C)C(=O)N([H])C(C(C)O)C(=O)N([H])[H])N([H])C(=O)C(CC1=CC2=CC=CC=C2C=C1)N([H])[H] | |
| External Links | ||
| DRUGBANK | DB06791 | |
| PubChem Compound | 71349 | |
| PDR | 1220 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
Not Available
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
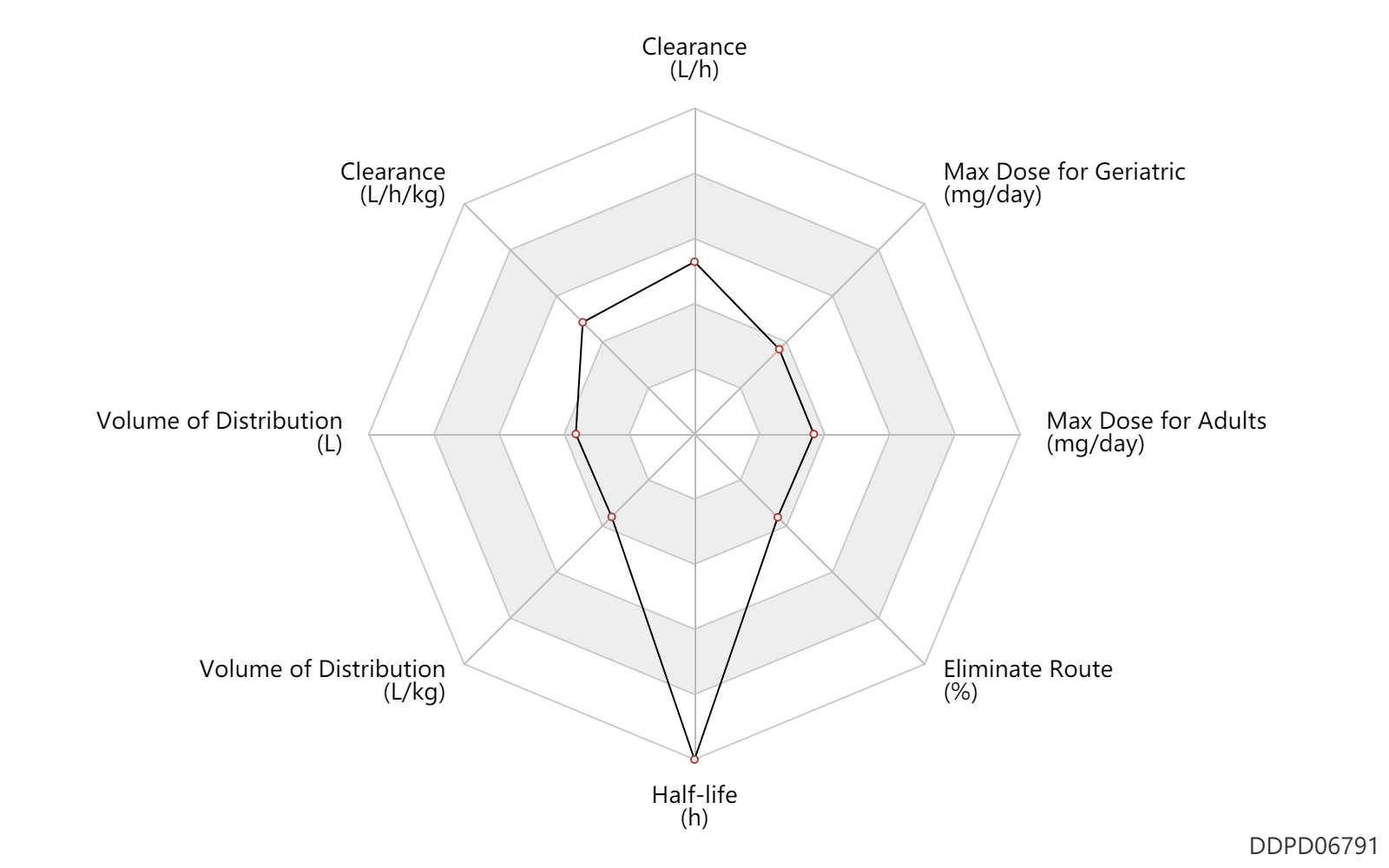
| Clearance | 23.1 | L/h | 23.1 | L/h | DRUGBANK | Clearance | 0.34 | L/h/kg | 5.7 | ml/min/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Volume of Distribution | 15.1 | L | 15.1 | L | DRUGBANK | Volume of Distribution | 0.20 | L/kg | 0.2 | L/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Half-life | 528.0 | h | ~22 | day | DRUGBANK | Half-life | 1.0 | h | 1.03 | h | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Eliminate Route | 5.0 | % | <5 | % | Urinary excretion; | DRUGBANK | Eliminate Route | 0.50 | % | <0.5 | % | Faeces excretion; Unchanged drug; | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Frequency | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Max dose for adults | 4.28571428571429 | mg/day | 120 | mg/dose | subcutaneous injection, SC | q4w | Somatuline Depot | lanreotide | PDR |
| Max dose for geriatric | 4.28571428571429 | mg/day | 120 | mg/dose | subcutaneous injection, SC | q4w | Somatuline Depot | lanreotide | PDR |