Basic Information
| Drug ID | DDPD06702 |

|
| Drug Name | Fesoterodine | |
| Molecular Weight | 411.5769 | |
| Molecular Formula | C26H37NO3 | |
| CAS Number | 286930-02-7 | |
| SMILES | CC(C)N(CC[C@H](C1=CC=CC=C1)C1=C(OC(=O)C(C)C)C=CC(CO)=C1)C(C)C | |
| External Links | ||
| DRUGBANK | DB06702 | |
| PubChem Compound | 6918558 | |
| PDR | 2593 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
Not Available
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
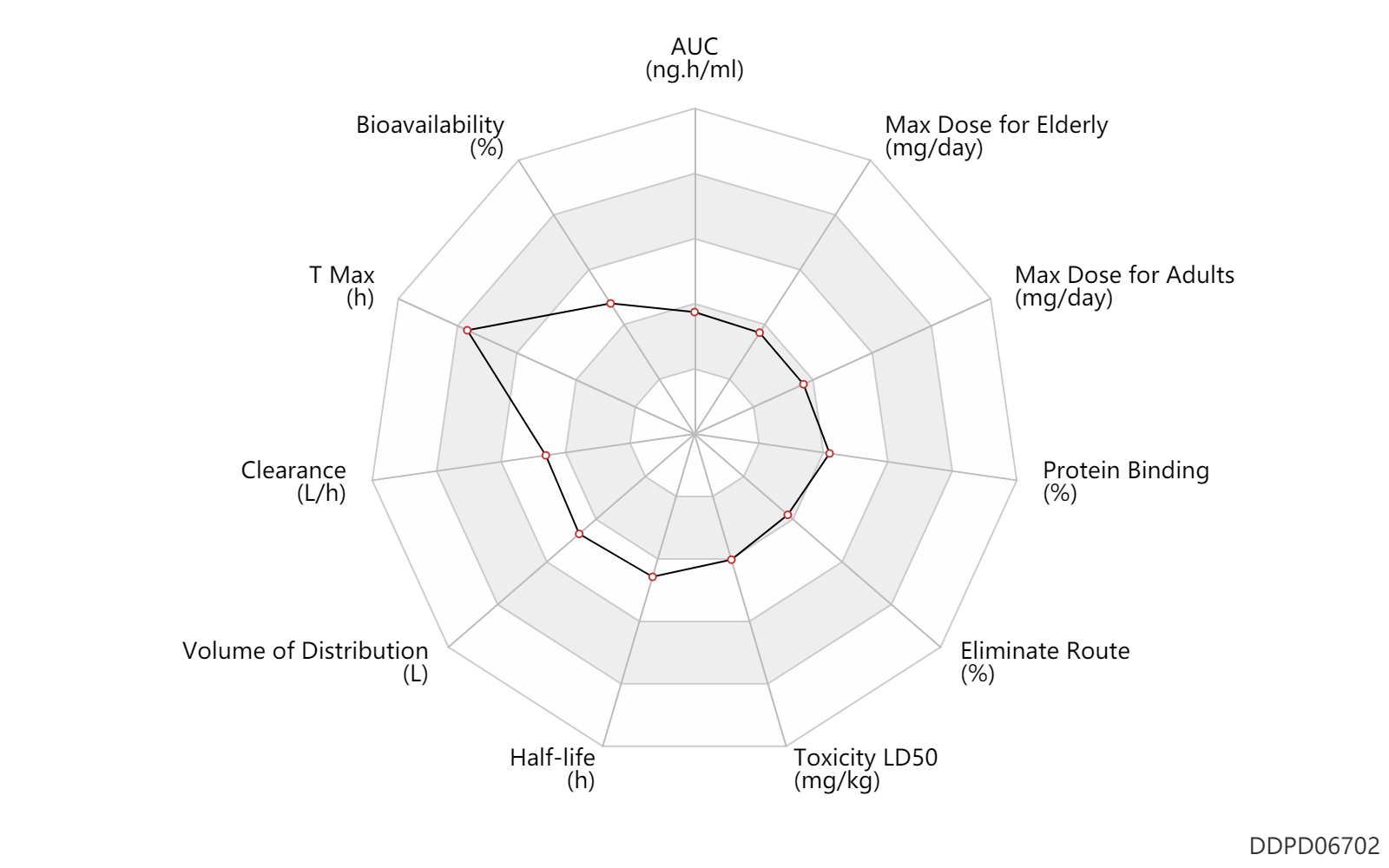
| AUC | 49.5 | ng.h/ml | 49.5 | ng.h/ml | DRUGBANK | |
| Bioavailability | 52.0 | % | 52 | % | DRUGBANK | |
| T Max | 5.0 | h | 5 | h | DRUGBANK | |
| Clearance | 14.4 | L/h | 14.4 | L/h | normal,healthy; | DRUGBANK |
| Volume of Distribution | 169.0 | L | 169.0 | L | intravenous injection, IV; | DRUGBANK |
| Half-life | 7.5 | h | 7-8 | h | Active metabolite; | DRUGBANK |
| Toxicity LD50 | 681.0 | mg/kg | ~681 | mg/kg | PO, oral; Rattus, Rat; | DRUGBANK | Toxicity LD50 | 316.0 | mg/kg | ~316 | mg/kg | PO, oral; mouse; | DRUGBANK |
| Toxicity NOAEL | 10.0 | mg/kg | 10.0 | mg/kg | intravenous injection, IV; Rattus, Rat; | DRUGBANK | Toxicity NOAEL | 10.0 | mg/kg | 10.0 | mg/kg | intravenous injection, IV; mouse; | DRUGBANK |
| Eliminate Route | 7.0 | % | 7 | % | Faeces excretion; | DRUGBANK |
| Protein Binding | 50.0 | % | 50 | % | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for adults | 8.0 | mg/day | 8 | mg/day | PO, oral | Toviaz | fesoterodine fumarate | PDR |
| Max dose for elderly | 8.0 | mg/day | 8 | mg/day | PO, oral | Toviaz | fesoterodine fumarate | PDR |