Basic Information
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
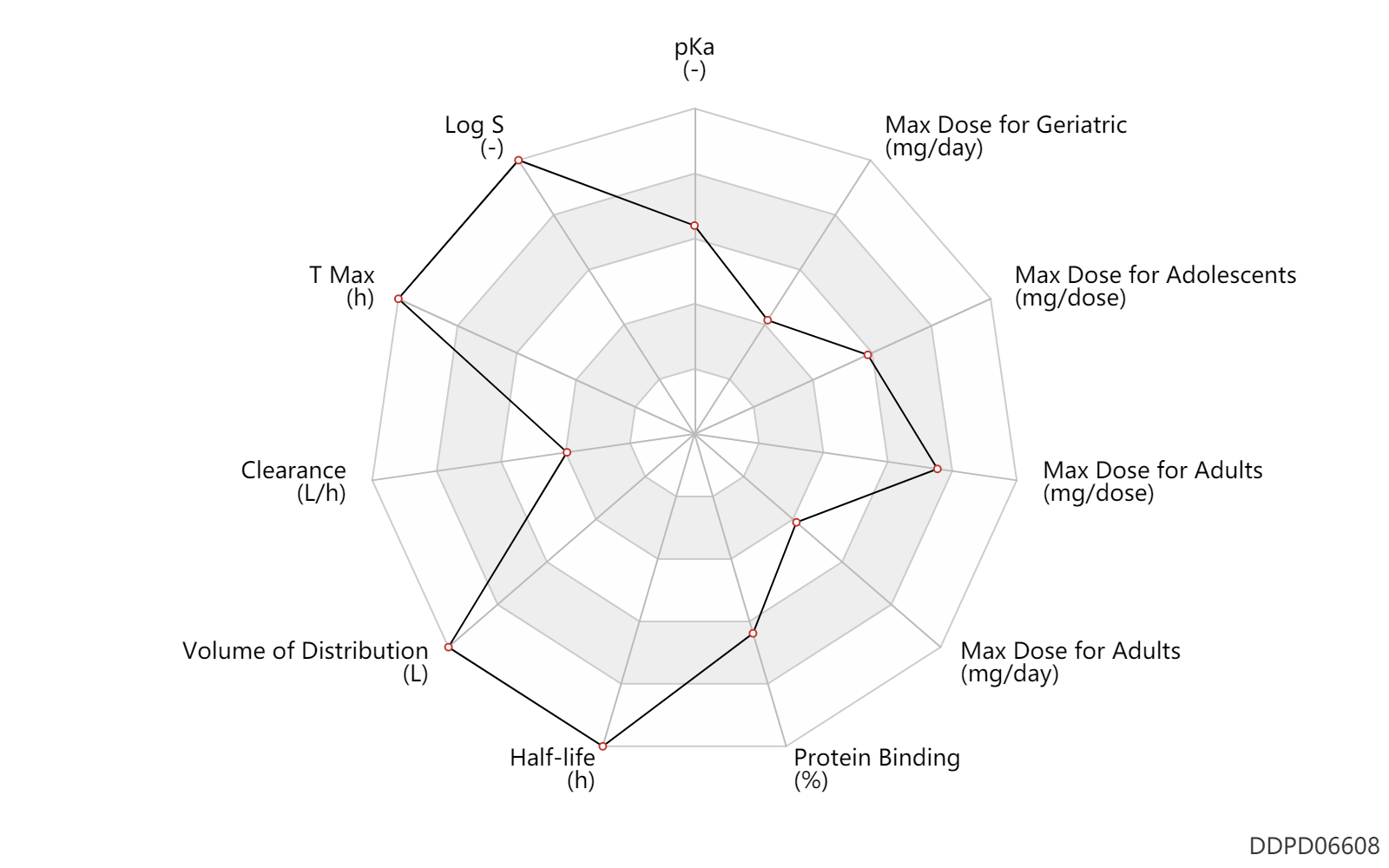
| pKa | 9.5 | - | 9.5 | - | Marella A. et al. (2013). Saudi Pharm J. Jan; 21 (1): 1-12 |
| Log S | 2.04 | - | 2.04 | - | Marella A. et al. (2013). Saudi Pharm J. Jan; 21 (1): 1-12 |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
| T Max | 13.8 | h | 13.8 | h | DRUGBANK | |
| Clearance | 6.0 | L/h | ~6 | L/h | DRUGBANK | |
| Volume of Distribution | 2560.0 | L | ~2560 | L | DRUGBANK | |
| Half-life | 336.0 | h | ~14 | day | DRUGBANK | |
| Protein Binding | 99.5 | % | ~99.5 | % | plasma proteins; human, homo sapiens; | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for adolescents | 300.0 | mg/dose | 300 | mg/dose | PO, oral | Krintafel | tafenoquine | PDR |
| Max dose for adults | 300.0 | mg/dose | 300 | mg/dose | PO, oral | Krintafel | tafenoquine | PDR |
| Max dose for adults | 200.0 | mg/day | 200 | mg/day | PO, oral | Krintafel | tafenoquine | PDR |
| Max dose for adults | 28.5714285714286 | mg/day | 200 | mg/week | PO, oral | Krintafel | tafenoquine | PDR |
| Max dose for geriatric | 300.0 | mg/dose | 300 | mg/dose | PO, oral | Krintafel | tafenoquine | PDR |
| Max dose for geriatric | 200.0 | mg/day | 200 | mg/day | PO, oral | Krintafel | tafenoquine | PDR |
| Max dose for geriatric | 28.5714285714286 | mg/day | 200 | mg/week | PO, oral | Krintafel | tafenoquine | PDR |