Basic Information
| Drug ID | DDPD06441 |

|
| Drug Name | Cangrelor | |
| Molecular Weight | 776.35 | |
| Molecular Formula | C17H25Cl2F3N5O12P3S2 | |
| CAS Number | 163706-06-7 | |
| SMILES | CSCCNC1=C2N=CN([C@@H]3O[C@H](COP(O)(=O)OP(O)(=O)C(Cl)(Cl)P(O)(O)=O)[C@@H](O)[C@H]3O)C2=NC(SCCC(F)(F)F)=N1 | |
| External Links | ||
| DRUGBANK | DB06441 | |
| PubChem Compound | 9854012 | |
| PDR | 3755 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
Not Available
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
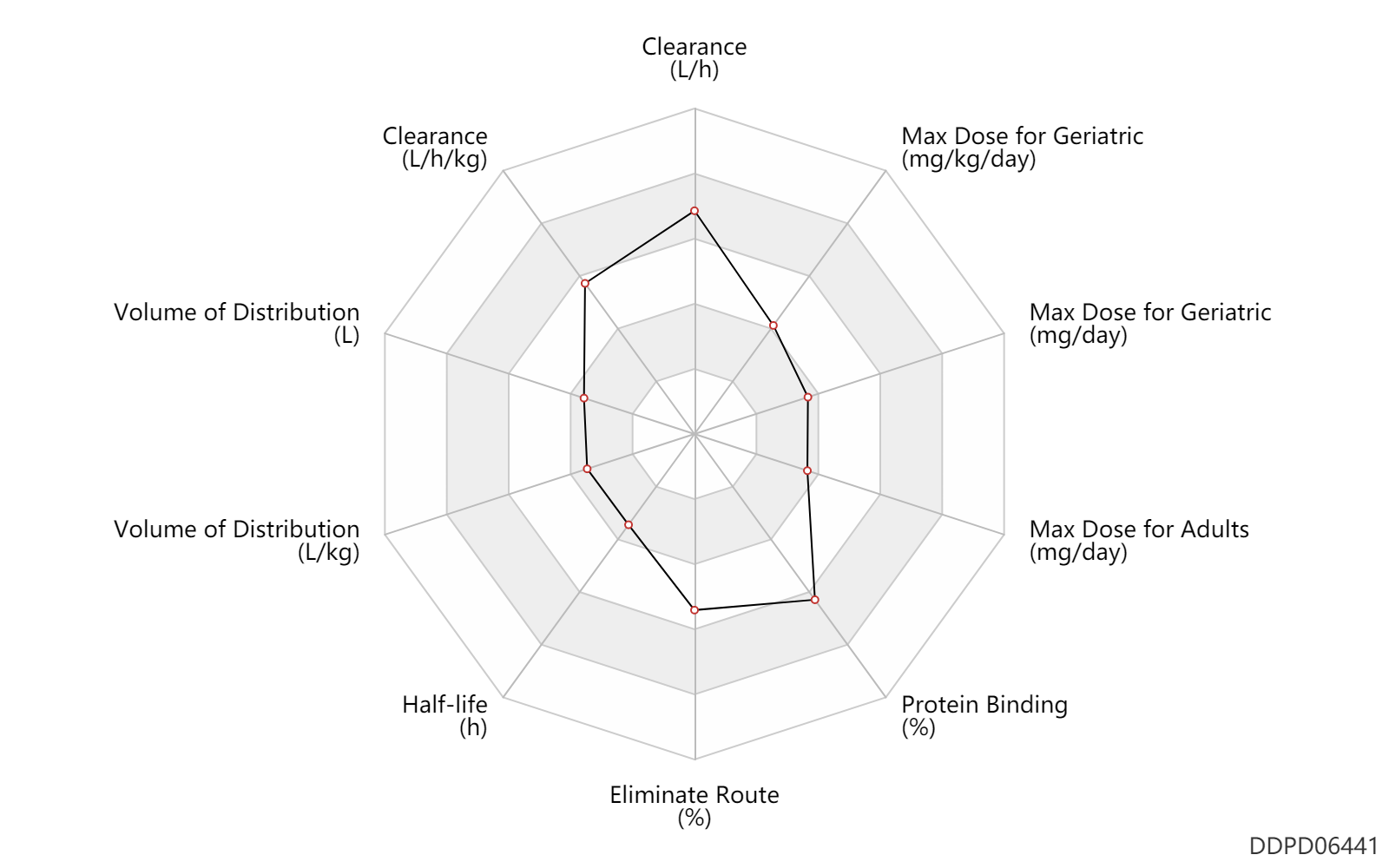
| Clearance | 43.2 | L/h | 43.2 | L/h | Average clearance; | DRUGBANK | Clearance | 0.54 | L/h/kg | 9.06 | ml/min/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Volume of Distribution | 3.9 | L | 3.9 | L | normal,healthy; | DRUGBANK | Volume of Distribution | 0.0350 | L/kg | 0.035 | L/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Half-life | 0.0750 | h | ~3-6 | min | elimination half-life; | DRUGBANK | Half-life | 0.14 | h | 0.14 | h | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Eliminate Route | 58.0 | % | 58 | % | Urinary excretion; intravenous injection, IV; | DRUGBANK | Eliminate Route | 35.0 | % | 35 | % | Faeces excretion; intravenous injection, IV; | DRUGBANK |
| Protein Binding | 97.5 | % | ~97-98 | % | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for adults | 0.03 | mg/day | 30 | mcg/day | intravenous injection, IV | Kengreal | cangrelor | PDR |
| Max dose for adults | 5.76 | mg/kg/day | 4 | mcg/kg/min | intravenous infusion, iv in drop | Kengreal | cangrelor | PDR |
| Max dose for geriatric | 0.03 | mg/day | 30 | mcg/day | intravenous injection, IV | Kengreal | cangrelor | PDR |
| Max dose for geriatric | 5.76 | mg/kg/day | 4 | mcg/kg/minute | intravenous infusion, iv in drop | Kengreal | cangrelor | PDR |