Basic Information
| Drug ID | DDPD06403 |

|
| Drug Name | Ambrisentan | |
| Molecular Weight | 378.428 | |
| Molecular Formula | C22H22N2O4 | |
| CAS Number | 177036-94-1 | |
| SMILES | COC([C@H](OC1=NC(C)=CC(C)=N1)C(O)=O)(C1=CC=CC=C1)C1=CC=CC=C1 | |
| External Links | ||
| DRUGBANK | DB06403 | |
| PubChem Compound | 6918493 | |
| PDR | 162 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
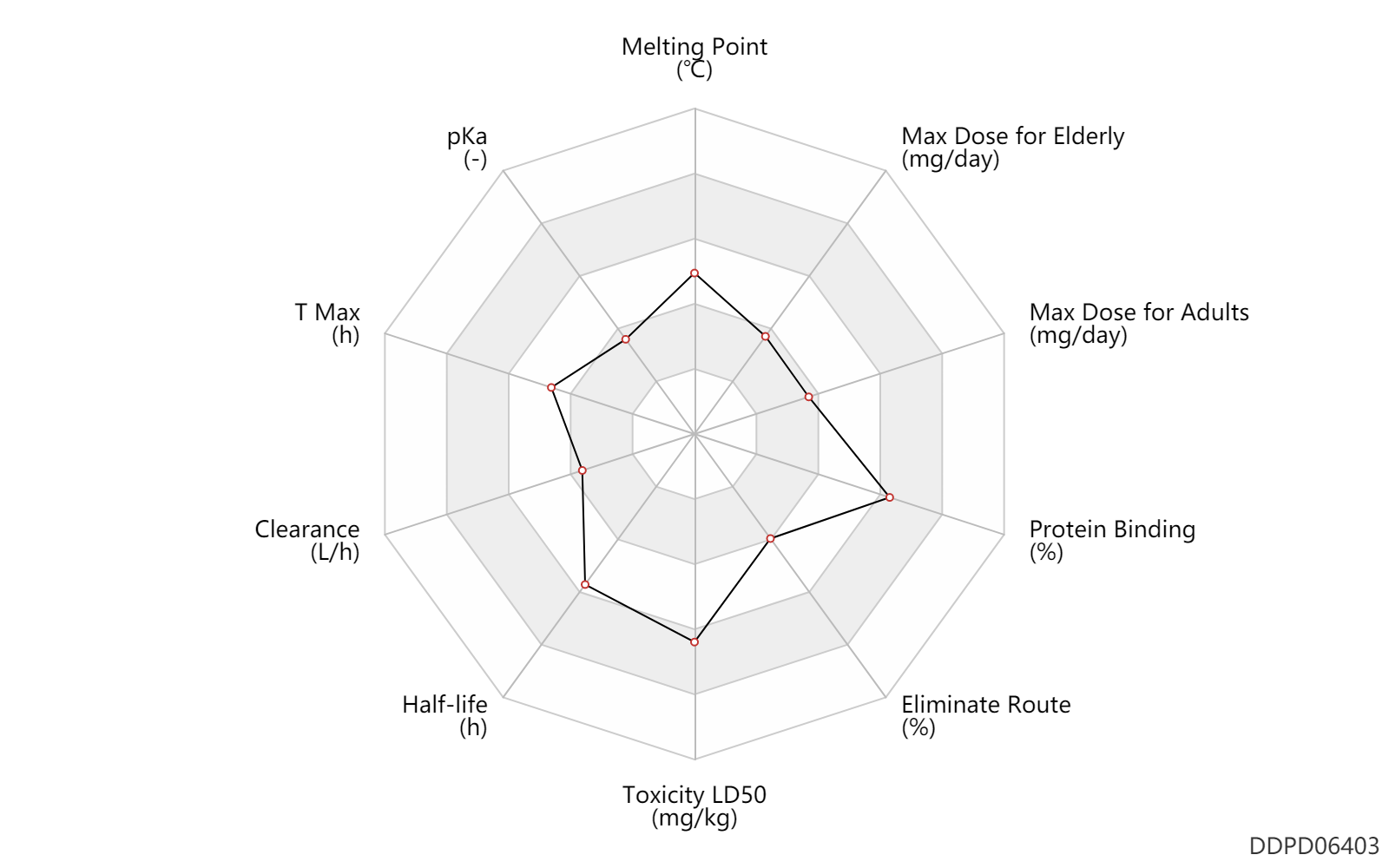
| Melting Point | 166.5 | ℃ | 165-168 | ℃ | # Riechers H, Albrecht HP, Amberg W, Baumann E, Bernard H, Bohm HJ, Klinge D, Kling A, Muller S, Raschack M, Unger L, Walker N, Wernet W: Discovery and optimization of a novel class of orally active nonpeptidic endothelin-A receptor antagonists. J Med Chem. 1996 May 24;39(11):2123-8. "Pubmed":http://www.ncbi.nlm.nih.gov/pubmed/8667356 |
| pKa | 4.0 | - | 4 | - | # FDA Label |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
| T Max | 2.0 | h | 2 | h | PO, oral; | DRUGBANK |
| Clearance | 2.3 | L/h | 38.0 | ml/min | Average clearance; PO, oral; normal,healthy; | DRUGBANK | Clearance | 1.1 | L/h | 19.0 | ml/min | Average clearance; PO, oral; pulmonary arterial hypertension; patients; | DRUGBANK |
| Half-life | 15.0 | h | 15 | h | DRUGBANK | |
| Toxicity LD50 | 3160.0 | mg/kg | >=3160 | mg/kg | Rattus, Rat; | DRUGBANK |
| Eliminate Route | 22.0 | % | ~22 | % | Urinary excretion; PO, oral; | DRUGBANK | Eliminate Route | 0.72 | % | ~0.72 | % | Urinary excretion; PO, oral; Unchanged drug; | DRUGBANK |
| Protein Binding | 99.0 | % | 99 | % | plasma proteins; | DRUGBANK | Protein Binding | 96.5 | % | 96.5 | % | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for adults | 10.0 | mg/day | 10 | mg/day | PO, oral | Letairis | ambrisentan | PDR |
| Max dose for elderly | 10.0 | mg/day | 10 | mg/day | PO, oral | Letairis | ambrisentan | PDR |