Basic Information
| Drug ID | DDPD06196 |

|
| Drug Name | Icatibant | |
| Molecular Weight | 1304.54 | |
| Molecular Formula | C59H89N19O13S | |
| CAS Number | 130308-48-4 | |
| SMILES | [H][C@]12C[C@]([H])(N(C(=O)[C@@]3([H])CC4=C(CN3C(=O)[C@H](CO)NC(=O)[C@H](CC3=CC=CS3)NC(=O)CNC(=O)[C@]3([H])C[C@@H](O)CN3C(=O)[C@@H]3CCCN3C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](N)CCCNC(N)=N)C=CC=C4)[C@@]1([H])CCCC2)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O | |
| External Links | ||
| DRUGBANK | DB06196 | |
| PubChem Compound | 71364 | |
| PDR | 1474 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
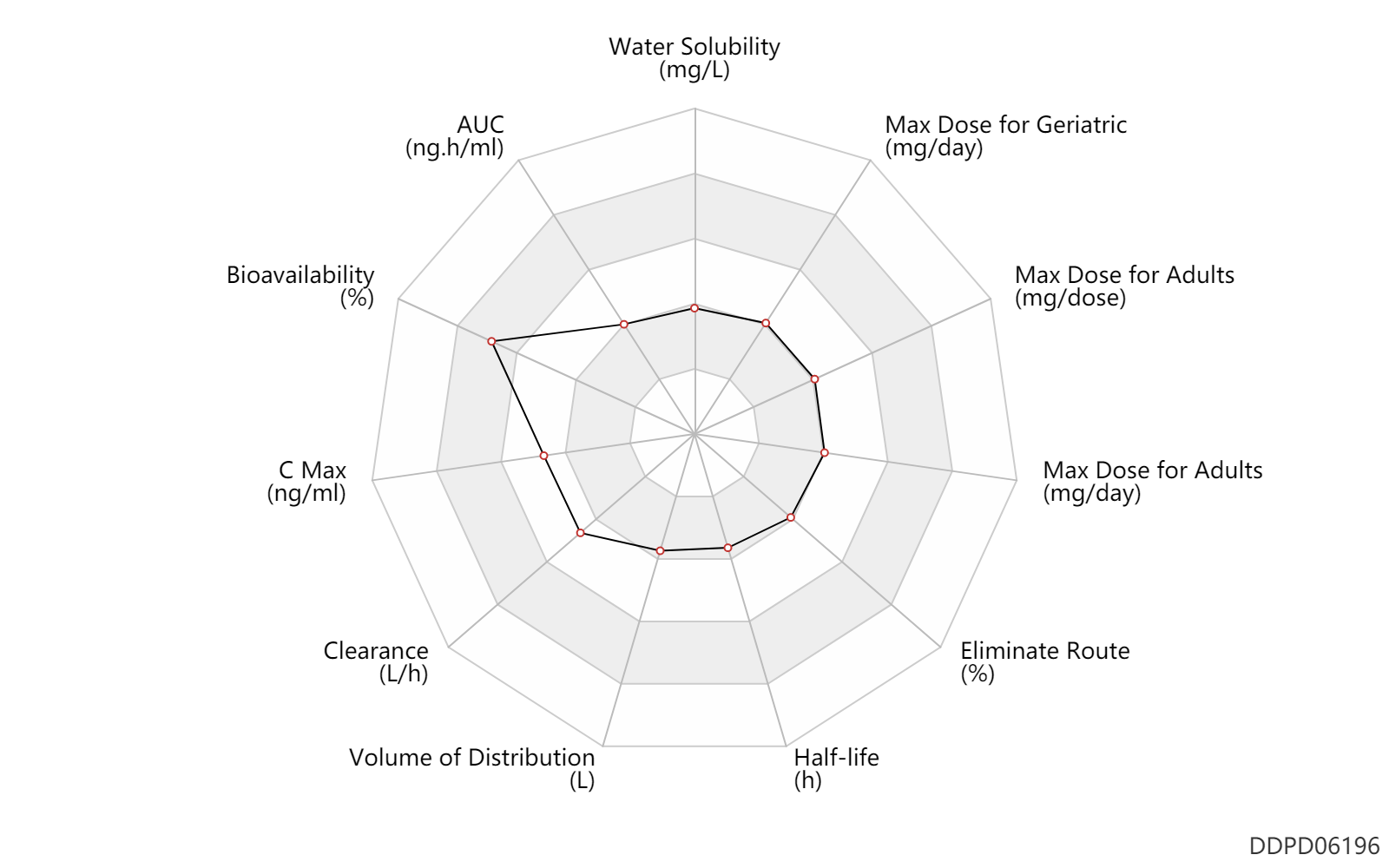
| Water Solubility | 1000.0 | mg/L | 1 | mg/ml | MSDS |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
| AUC | 2165.0 | ng.h/ml | 2165±568 | ng.h/ml | subcutaneous injection, SC; | DRUGBANK |
| Bioavailability | 97.0 | % | ~97 | % | subcutaneous injection, SC; | DRUGBANK |
| C Max | 974.0 | ng/ml | 974±280 | ng/ml | subcutaneous injection, SC; | DRUGBANK |
| Clearance | 14.7 | L/h | 245±58 | ml/min | Plasma clearance; subcutaneous injection, SC; | DRUGBANK |
| Volume of Distribution | 29.0 | L | 29.0 ± 8.7 | L | subcutaneous injection, SC; | DRUGBANK |
| Half-life | 1.4 | h | 1.4±0.4 | h | elimination half-life; subcutaneous injection, SC; | DRUGBANK |
| Eliminate Route | 10.0 | % | <10 | % | Urinary excretion; Unchanged drug; | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for adults | 30.0 | mg/dose | 30 | mg/dose | subcutaneous injection, SC | Firazyr | icatibant | PDR |
| Max dose for adults | 90.0 | mg/day | 90 | mg/day | subcutaneous injection, SC | Firazyr | icatibant | PDR |
| Max dose for geriatric | 30.0 | mg/dose | 30 | mg/dose | subcutaneous injection, SC | Firazyr | icatibant | PDR |
| Max dose for geriatric | 90.0 | mg/day | 90 | mg/day | subcutaneous injection, SC | Firazyr | icatibant | PDR |