Basic Information
Experimental Physicochemical Property
Not Available
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
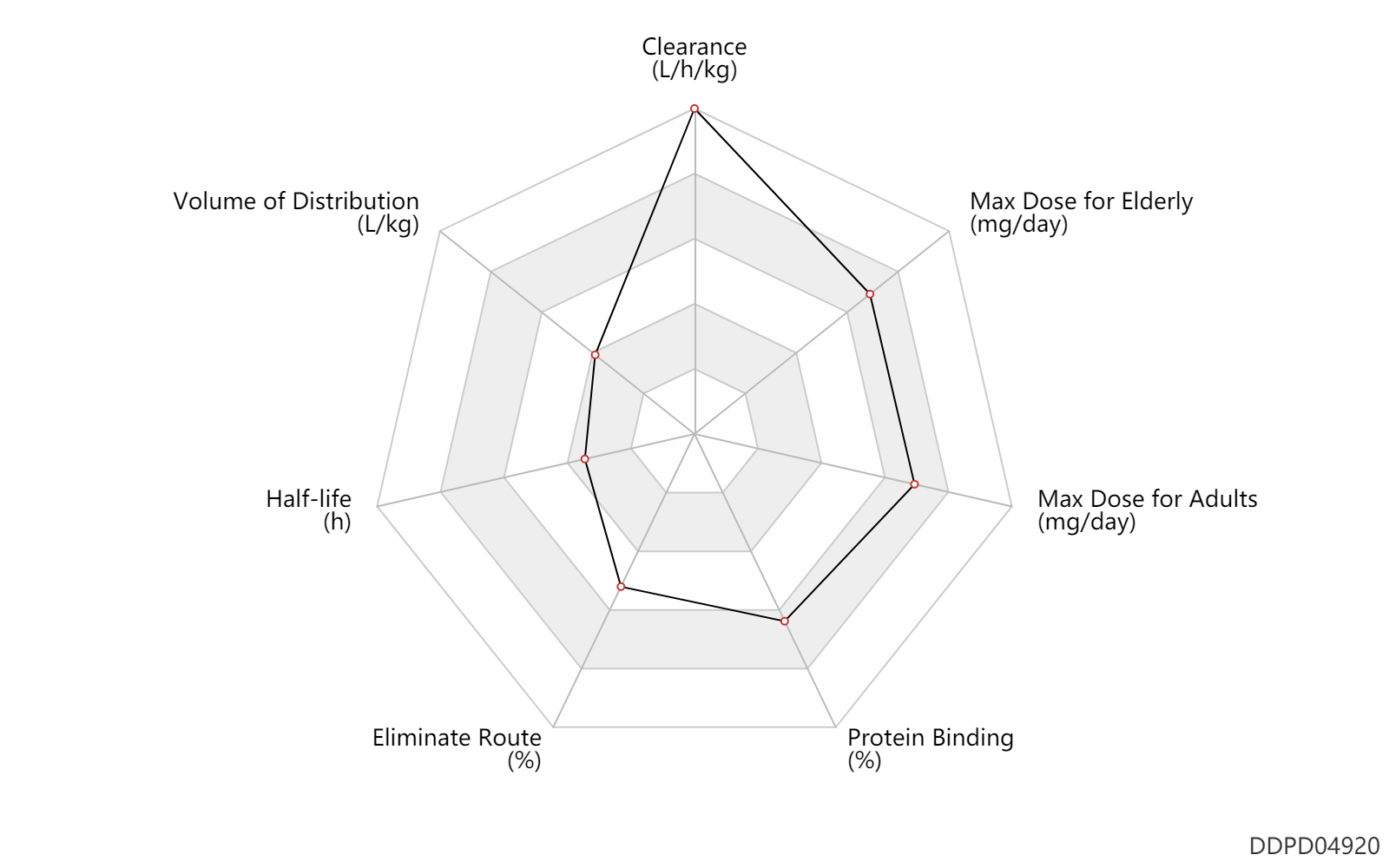
| Clearance | 8.5 | L/h/kg | 142 | ml/min/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Volume of Distribution | 0.58 | L/kg | 0.58 | L/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Half-life | 0.0167 | h | 1 | min | DRUGBANK | Half-life | 0.30 | h | 0.3 | h | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Eliminate Route | 68.5 | % | 63-74 | % | Urinary excretion; | DRUGBANK | Eliminate Route | 14.5 | % | 7-22 | % | Faeces excretion; | DRUGBANK |
| Protein Binding | 99.5 | % | >99.5 | % | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for adults | 768.0 | mg/day | 32 | mg/h | intravenous injection, IV | Cleviprex | clevidipine | PDR |
| Max dose for adults | 1000.0 | mg/day | 1000 | mg/day | intravenous injection, IV | Cleviprex | clevidipine | PDR |
| Max dose for adults | 504.0 | mg/day | 21 | mg/h | intravenous injection, IV | Cleviprex | clevidipine | PDR |
| Max dose for elderly | 32.0 | mg/hr | 32 | mg/hr | intravenous injection, IV | Cleviprex | clevidipine | PDR |
| Max dose for elderly | 1000.0 | mg/day | 1000 | mg/day | intravenous injection, IV | Cleviprex | clevidipine | PDR |
| Max dose for elderly | 21.0 | mg/hr | 21 | mg/hr | intravenous injection, IV | Cleviprex | clevidipine | PDR |