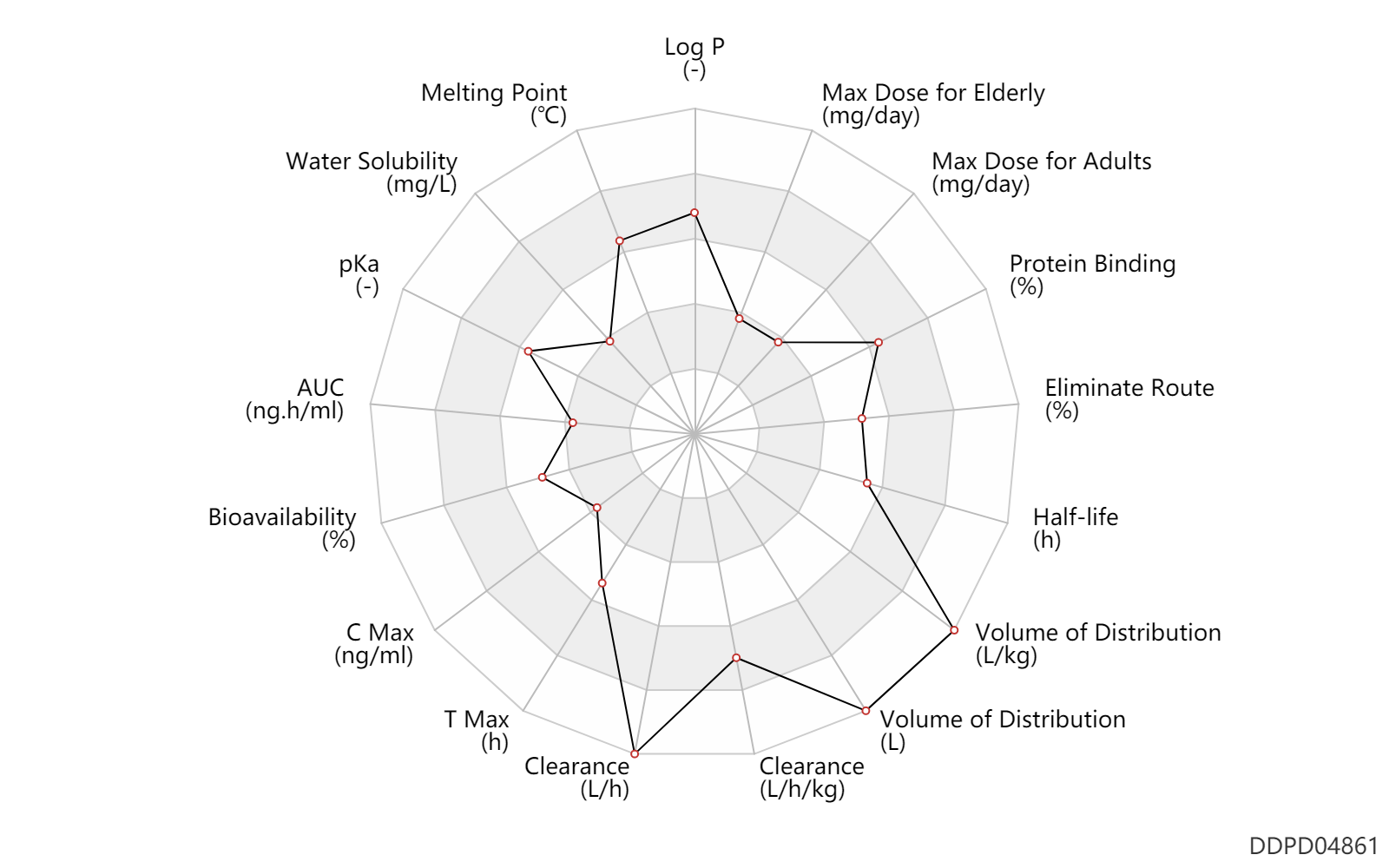
| Property Name |
Property Value |
Unit |
Raw Value |
Raw Unit |
Annotation |
Reference |
| AUC |
13.8 |
ng.h/ml |
13.78±15.27 |
ng.h/ml |
Raceme D; |
DRUGBANK |
AUC |
27.7 |
ng.h/ml |
27.72±15.32 |
ng.h/ml |
Raceme L; |
DRUGBANK |
AUC |
41.5 |
ng.h/ml |
41.50±29.76 |
ng.h/ml |
Raceme D/L; |
DRUGBANK |
AUC |
396.8 |
ng.h/ml |
396.78±297.94 |
ng.h/ml |
Derivative; |
DRUGBANK |
| Bioavailability |
54.0 |
% |
12-96 |
% |
PO, oral; poor metabolizers, PM; extensive metabolizers, EM; |
DRUGBANK |
| C Max |
2.8 |
ng/ml |
2.75±1.55 |
ng/ml |
Raceme D; |
DRUGBANK |
C Max |
5.3 |
ng/ml |
5.29±2.06 |
ng/ml |
Raceme L; |
DRUGBANK |
C Max |
8.0 |
ng/ml |
8.02±3.47 |
ng/ml |
Raceme D/L; |
DRUGBANK |
C Max |
68.3 |
ng/ml |
68.34±44.68 |
ng/ml |
Derivative; |
DRUGBANK |
| T Max |
2.8 |
h |
1.5-4 |
h |
PO, oral; poor metabolizers, PM; extensive metabolizers, EM; |
DRUGBANK |
| Clearance |
1241.6 |
L/h |
1241.63±749.77 |
L/h |
|
DRUGBANK |
Clearance |
435.5 |
L/h |
435.53±180.93 |
L/h |
|
DRUGBANK |
Clearance |
635.3 |
L/h |
635.31±300.25 |
L/h |
|
DRUGBANK |
Clearance |
0.84 |
L/h/kg |
14 |
ml/min/kg |
intravenous injection, IV; human, homo sapiens; |
Human Intravenous Pharmacokinetic Dataset |
| Volume of Distribution |
10290.8 |
L |
10290.81±3911.72 |
L |
Apparent volume of distribution; |
DRUGBANK |
Volume of Distribution |
8066.7 |
L |
8066.66±4055.50 |
L |
Apparent volume of distribution; |
DRUGBANK |
Volume of Distribution |
10423.4 |
L |
10423.42±6796.50 |
L |
Apparent volume of distribution; |
DRUGBANK |
Volume of Distribution |
11.0 |
L/kg |
11 |
L/kg |
intravenous injection, IV; human, homo sapiens; |
Human Intravenous Pharmacokinetic Dataset |
| Half-life |
12.0 |
h |
12 |
h |
Raceme D; extensive metabolizers, EM; |
DRUGBANK |
Half-life |
19.0 |
h |
19 |
h |
Raceme D; poor metabolizers, PM; |
DRUGBANK |
Half-life |
10.0 |
h |
10 |
h |
intravenous injection, IV; human, homo sapiens; |
Human Intravenous Pharmacokinetic Dataset |
| Eliminate Route |
38.0 |
% |
38 |
% |
Urinary excretion; extensive metabolizers, EM; |
DRUGBANK |
Eliminate Route |
44.0 |
% |
44 |
% |
Faeces excretion; extensive metabolizers, EM; |
DRUGBANK |
Eliminate Route |
67.0 |
% |
67 |
% |
Urinary excretion; poor metabolizers, PM; |
DRUGBANK |
Eliminate Route |
13.0 |
% |
13 |
% |
Faeces excretion; poor metabolizers, PM; |
DRUGBANK |
| Protein Binding |
98.0 |
% |
98 |
% |
plasma proteins; |
DRUGBANK |