Basic Information
| Drug ID | DDPD04844 |

|
| Drug Name | Tetrabenazine | |
| Molecular Weight | 317.4226 | |
| Molecular Formula | C19H27NO3 | |
| CAS Number | 58-46-8 | |
| SMILES | COC1=C(OC)C=C2C3CC(=O)C(CC(C)C)CN3CCC2=C1 | |
| External Links | ||
| DRUGBANK | DB04844 | |
| PubChem Compound | 6018 | |
| PDR | 298 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
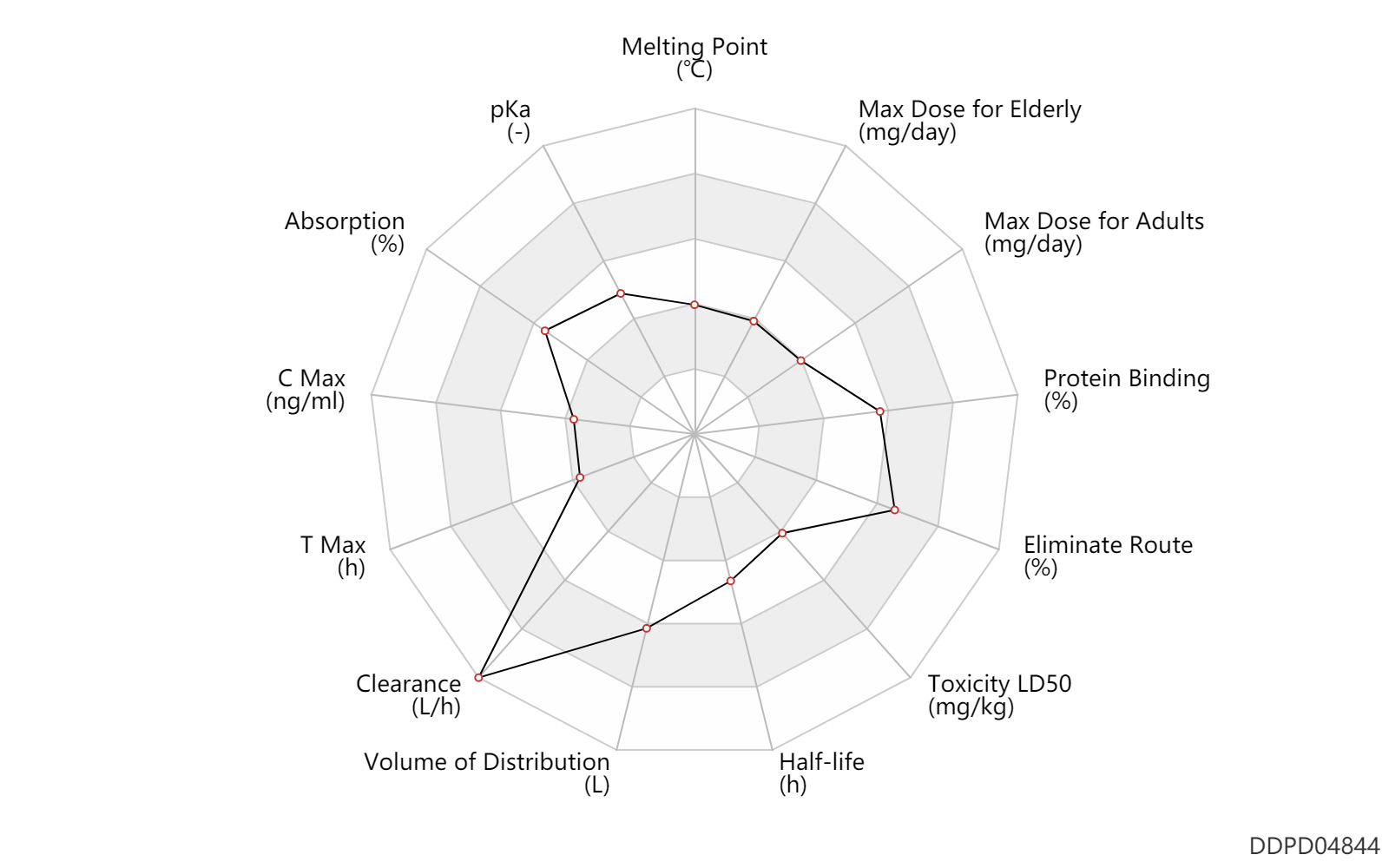
| Melting Point | 126.0 | ℃ | 126 | ℃ | MSDS |
| pKa | 6.51 | - | 6.51 | - | FDA label |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
| Absorption | 75.0 | % | >75 | % | PO, oral; | DRUGBANK |
| C Max | 4.8 | ng/ml | 4.8 | ng/ml | PO, oral; Huntington's disease; tardive dyskinesia; | DRUGBANK |
| T Max | 1.2 | h | 69 | min | PO, oral; Huntington's disease; tardive dyskinesia; | DRUGBANK |
| Clearance | 100.2 | L/h | 1.67 | L/min | intravenous injection, IV; | DRUGBANK |
| Volume of Distribution | 385.0 | L | 385.0 | L | intravenous injection, IV; at steady state; | DRUGBANK |
| Half-life | 7.0 | h | 7 | h | distribution half-life; | DRUGBANK | Half-life | 5.0 | h | 5 | h | elimination half-life; | DRUGBANK | Half-life | 12.0 | h | 12 | h | elimination half-life; | DRUGBANK |
| Toxicity LD50 | 550.0 | mg/kg | 550.0 | mg/kg | PO, oral; mouse; | DRUGBANK |
| Eliminate Route | 75.0 | % | 75 | % | Urinary excretion; PO, oral; | DRUGBANK |
| Protein Binding | 85.0 | % | 82-88 | % | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for adults | 100.0 | mg/day | 100 | mg/day | PO, oral | Xenazine | tetrabenazine | PDR |
| Max dose for adults | 50.0 | mg/day | 50 | mg/day | PO, oral | Xenazine | tetrabenazine | PDR |
| Max dose for elderly | 100.0 | mg/day | 100 | mg/day | PO, oral | Xenazine | tetrabenazine | PDR |
| Max dose for elderly | 50.0 | mg/day | 50 | mg/day | PO, oral | Xenazine | tetrabenazine | PDR |