Basic Information
| Drug ID | DDPD04839 |

|
| Drug Name | Cyproterone acetate | |
| Molecular Weight | 416.938 | |
| Molecular Formula | C24H29ClO4 | |
| CAS Number | 427-51-0 | |
| SMILES | [H][C@@]12C[C@]1([H])[C@@]1(C)C(=CC2=O)C(Cl)=C[C@@]2([H])[C@]3([H])CC[C@](OC(C)=O)(C(C)=O)[C@@]3(C)CC[C@]12[H] | |
| External Links | ||
| DRUGBANK | DB04839 | |
| T3DB | T3D4747 | |
| PubChem Compound | 9880 | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
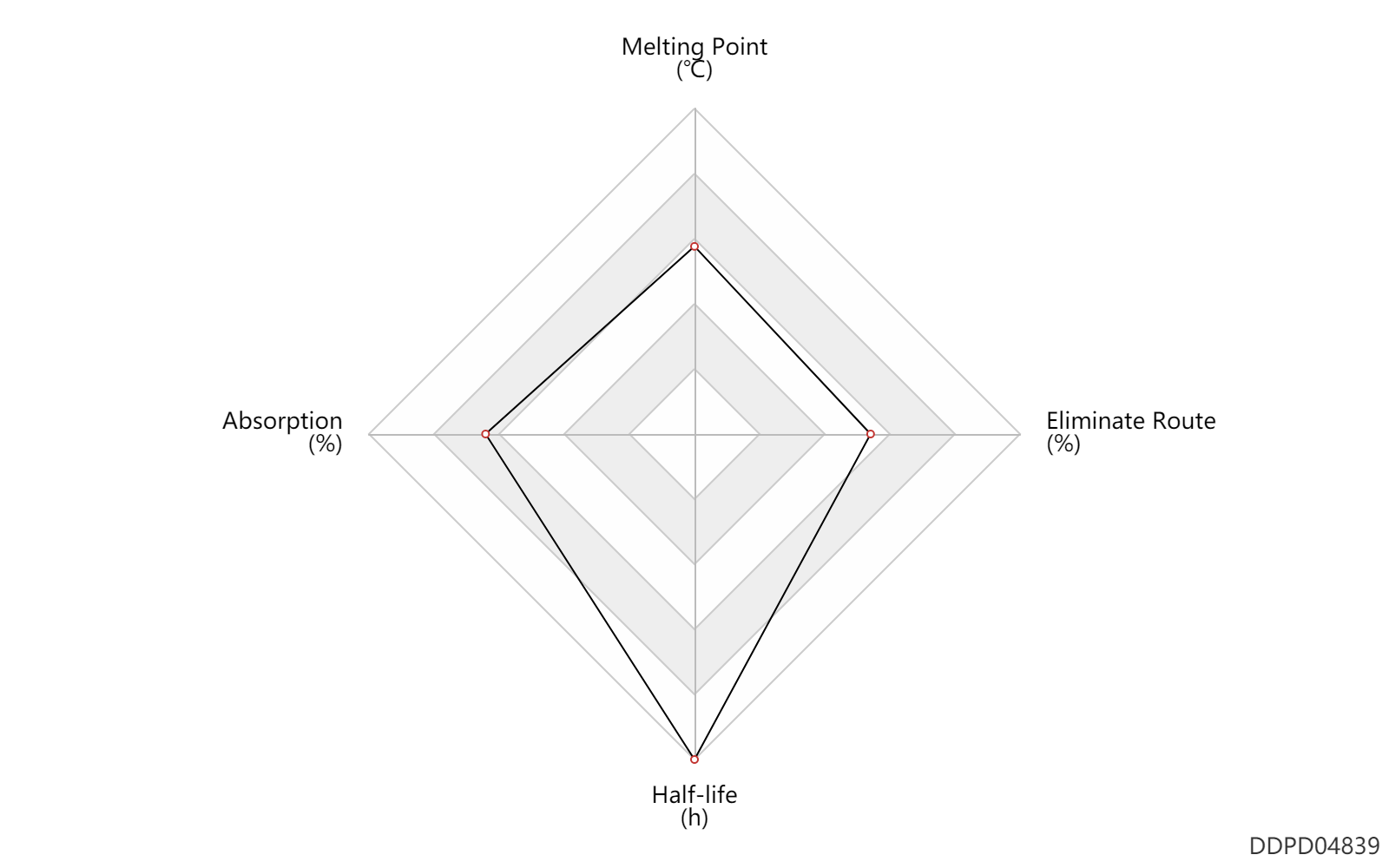
| Melting Point | 200.5 | ℃ | 200-201 | ℃ | Wiechert, R.; U S . Patent 3,234,093; February 8, 1966; assigned to Schering AG, Germany. |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
| Absorption | 100.0 | % | 100 | % | DRUGBANK | |
| Half-life | 38.0 | h | 38 | h | elimination half-life; PO, oral; | DRUGBANK | Half-life | 96.0 | h | 96 | h | elimination half-life; IM,intramuscular injection; | DRUGBANK |
| Eliminate Route | 60.0 | % | ~60 | % | Bile excretion; | DRUGBANK | Eliminate Route | 33.0 | % | 33 | % | Urinary excretion; | DRUGBANK |
Maximum Dosage
Not Available