Basic Information
| Drug ID | DDPD03904 |

|
| Drug Name | Urea | |
| Molecular Weight | 60.0553 | |
| Molecular Formula | CH4N2O | |
| CAS Number | 57-13-6 | |
| SMILES | NC(N)=O | |
| External Links | ||
| DRUGBANK | DB03904 | |
| PubChem Compound | 1176 | |
| PDR | 3547 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
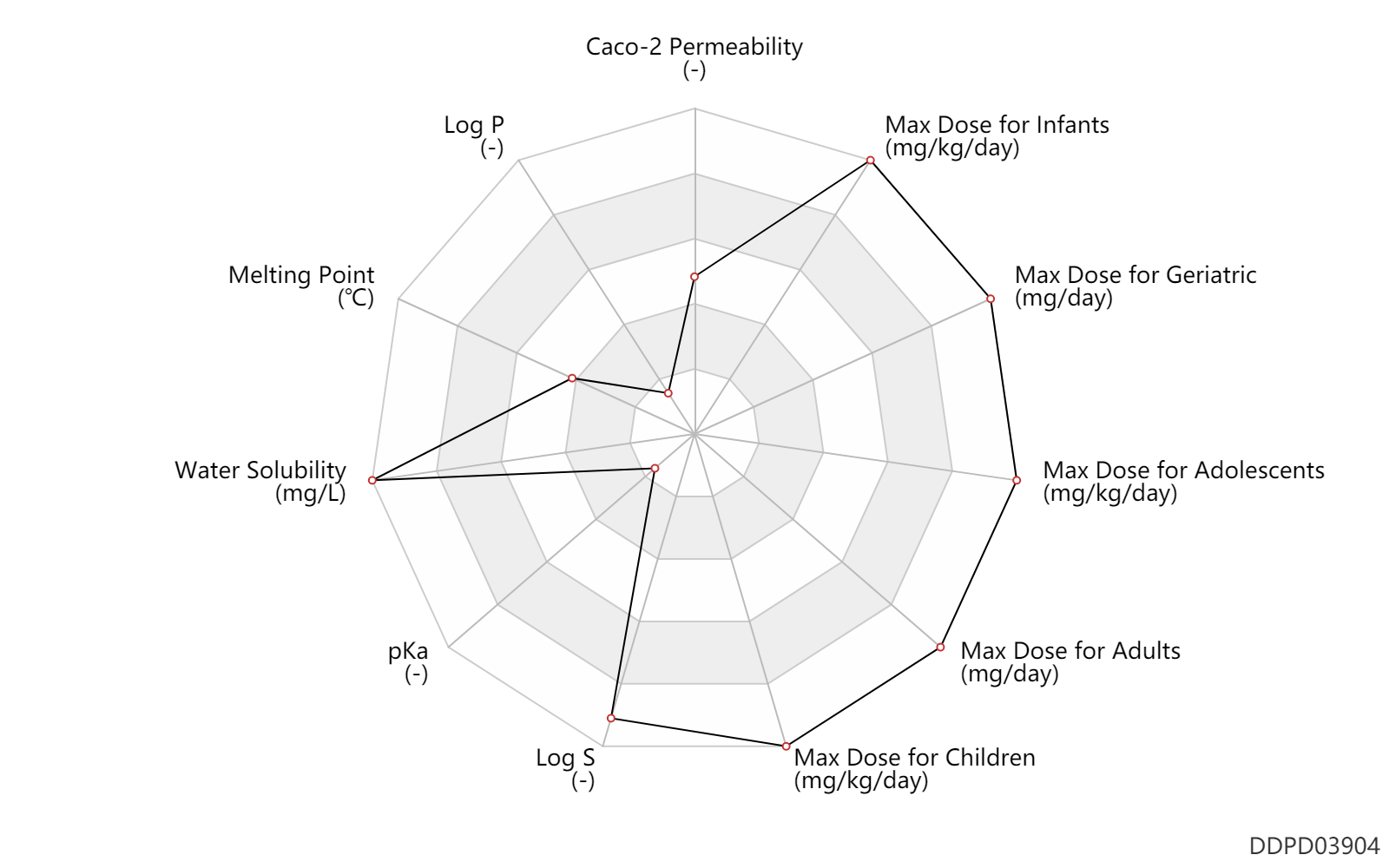
| Caco-2 Permeability | -5.34 | - | -5.34 | - | ADME Research, USCD |
| Log P | -2.11 | - | -2.11 | - | HANSCH,C ET AL. (1995) |
| Melting Point | 132.7 | ℃ | 132.7 | ℃ | PhysProp |
| Water Solubility | 545000.0 | mg/L | 545000 | mg/L | YALKOWSKY,SH 1989) |
| pKa | 0.1 | - | 0.1 | - | PERRIN,DD (1965) |
| Log S | 0.96 | - | 0.96 | - | ADME Research, USCD |
Pharmacokinetic/ Toxicokinetic Property
Not Available
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for infants | 2000.0 | mg/kg/day | 2 | g/kg/day | PO, oral | Hydro 35 | urea | PDR |
| Max dose for children | 2.0 | application/day | 2 | application/day | skin/dermal | Hydro 35 | urea | PDR |
| Max dose for children | 2000.0 | mg/kg/day | 2 | g/kg/day | PO, oral | Hydro 35 | urea | PDR |
| Max dose for adolescents | 2.0 | application/day | 2 | application/day | skin/dermal | Hydro 35 | urea | PDR |
| Max dose for adolescents | 2000.0 | mg/kg/day | 2 | g/kg/day | PO, oral | Hydro 35 | urea | PDR |
| Max dose for adults | 2.0 | appLication/day | 2 | appLication/day | skin/dermal | Hydro 35 | urea | PDR |
| Max dose for adults | 180000.0 | mg/day | 180 | g/day | PO, oral | Hydro 35 | urea | PDR |
| Max dose for geriatric | 2.0 | application/day | 2 | application/day | skin/dermal | Hydro 35 | urea | PDR |
| Max dose for geriatric | 180000.0 | mg/day | 180 | g/day | PO, oral | Hydro 35 | urea | PDR |