Basic Information
| Drug ID | DDPD01610 |

|
| Drug Name | Valganciclovir | |
| Molecular Weight | 354.3617 | |
| Molecular Formula | C14H22N6O5 | |
| CAS Number | 175865-60-8 | |
| SMILES | CC(C)[C@H](N)C(=O)OCC(CO)OCN1C=NC2=C1NC(N)=NC2=O | |
| External Links | ||
| DRUGBANK | DB01610 | |
| PubChem Compound | 64147 | |
| PDR | 2146 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
Not Available
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Factor | Reference |
|---|---|---|---|---|---|---|---|
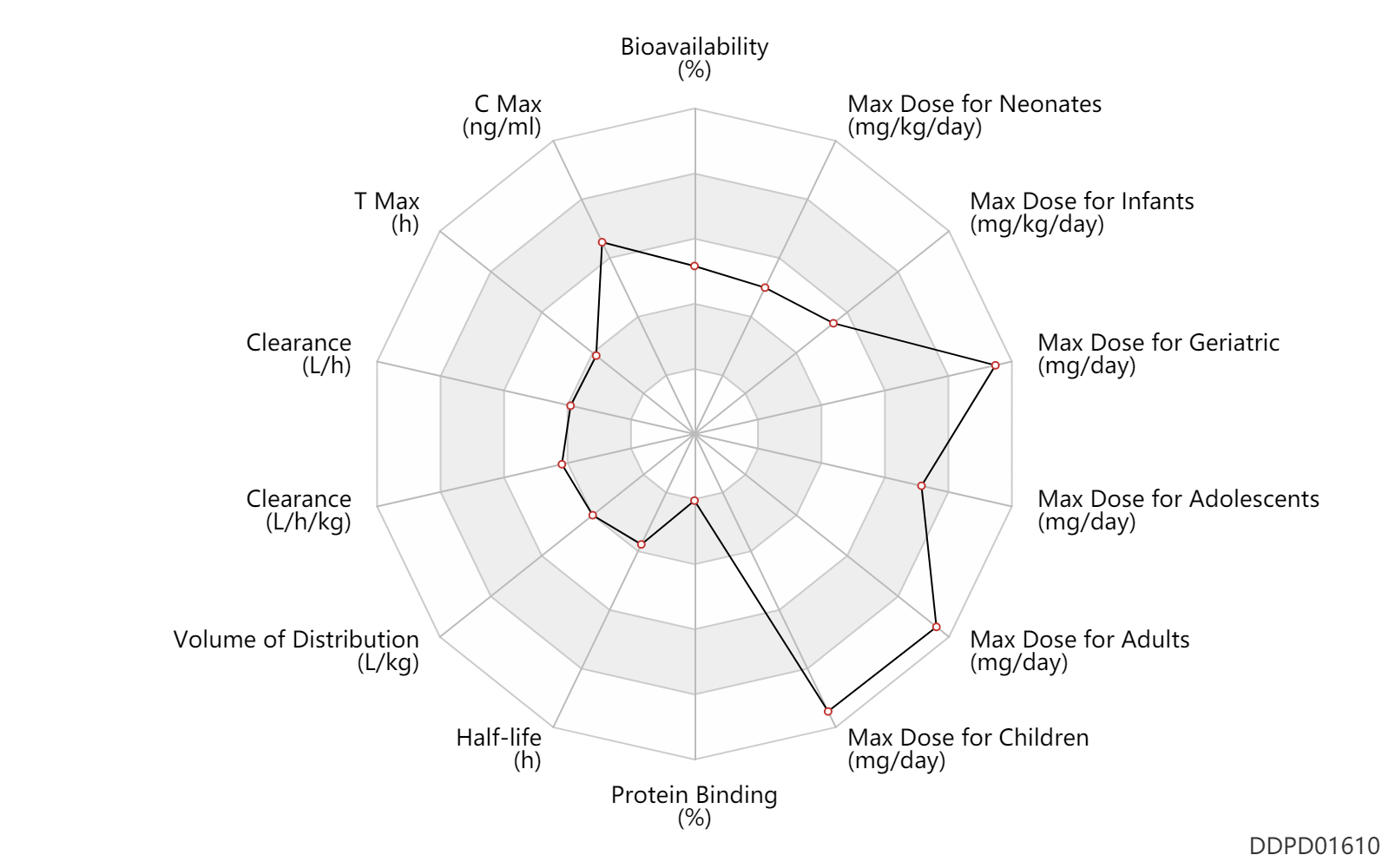
| Bioavailability | 60.0 | % | 60 | % | Tablet, PO, oral; food; | DRUGBANK | Bioavailability | 61.0 | % | 61±9 | % | PO, oral; | The Pharmacological Basis of Therapeutics |
| C Max | 200.0 | ng/ml | 0.20±0.7 | mcg/ml | PO, oral; food; | The Pharmacological Basis of Therapeutics | C Max | 5600.0 | ng/ml | 5.6±1.5 | mcg/ml | PO, oral; Drug form; food; | The Pharmacological Basis of Therapeutics |
| T Max | 0.50 | h | 0.5±0.3 | h | PO, oral; food; | The Pharmacological Basis of Therapeutics | T Max | 2.0 | h | 1-3 | h | PO, oral; Drug form; food; | The Pharmacological Basis of Therapeutics |
| Clearance | 0.18 | L/h/kg | 3.07±0.64 | ml/min/kg | intravenous injection, IV; | DRUGBANK | Clearance | 5.3 | L/h | 5.3±0.9 | L/h | mild renal function; patients; | DRUGBANK |
| Volume of Distribution | 0.70 | L/kg | 0.703±0.134 | L/kg | DRUGBANK | ||
| Half-life | 4.1 | h | ~4.08 | h | RD, renal impairment, Renal disease,including uremia ↑ ; | DRUGBANK | Half-life | 0.50 | h | 0.5±0.2 | h | PO, oral; Male, men; Female, women; viral infections; | The Pharmacological Basis of Therapeutics | Half-life | 3.7 | h | 3.7±0.6 | h | Metabolite; PO, oral; Male, men; Female, women; viral infections; | RD, renal impairment, Renal disease,including uremia ↑ ; | The Pharmacological Basis of Therapeutics | Half-life | 0.50 | h | 0.5 | h | elimination half-life; | The Pharmacological Basis of Therapeutics |
| Protein Binding | 1.5 | % | 1-2 | % | plasma proteins; | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for neonates | 32.0 | mg/kg/day | 32 | mg/kg/day | PO, oral | Valcyte | valganciclovir hydrochloride | PDR |
| Max dose for infants | 32.0 | mg/kg/day | 32 | mg/kg/day | PO, oral | Valcyte | valganciclovir hydrochloride | PDR |
| Max dose for children | 900.0 | mg/day | 900 | mg/day | PO, oral | Valcyte | valganciclovir hydrochloride | PDR |
| Max dose for children | 1800.0 | mg/day | 1800 | mg/day | PO, oral | Valcyte | valganciclovir hydrochloride | PDR |
| Max dose for adolescents | 900.0 | mg/day | 900 | mg/day | PO, oral | Valcyte | valganciclovir hydrochloride | PDR |
| Max dose for adolescents | 1800.0 | mg/day | 1800 | mg/day | PO, oral | Valcyte | valganciclovir hydrochloride | PDR |
| Max dose for adolescents | 900.0 | mg/day | 900 | mg/day | PO, oral | Valcyte | valganciclovir hydrochloride | PDR |
| Max dose for adolescents | 1800.0 | mg/day | 1800 | mg/day | PO, oral | Valcyte | valganciclovir hydrochloride | PDR |
| Max dose for adults | 900.0 | mg/day | 900 | mg/day | PO, oral | Valcyte | valganciclovir hydrochloride | PDR |
| Max dose for adults | 1800.0 | mg/day | 1800 | mg/day | PO, oral | Valcyte | valganciclovir hydrochloride | PDR |
| Max dose for geriatric | 900.0 | mg/day | 900 | mg/day | PO, oral | Valcyte | valganciclovir hydrochloride | PDR |
| Max dose for geriatric | 1800.0 | mg/day | 1800 | mg/day | PO, oral | Valcyte | valganciclovir hydrochloride | PDR |