Basic Information
| Drug ID | DDPD01591 |

|
| Drug Name | Solifenacin | |
| Molecular Weight | 362.473 | |
| Molecular Formula | C23H26N2O2 | |
| CAS Number | 242478-37-1 | |
| SMILES | O=C(O[C@H]1CN2CCC1CC2)N1CCC2=CC=CC=C2[C@@H]1C1=CC=CC=C1 | |
| External Links | ||
| DRUGBANK | DB01591 | |
| PubChem Compound | 154059 | |
| PDR | 2420 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
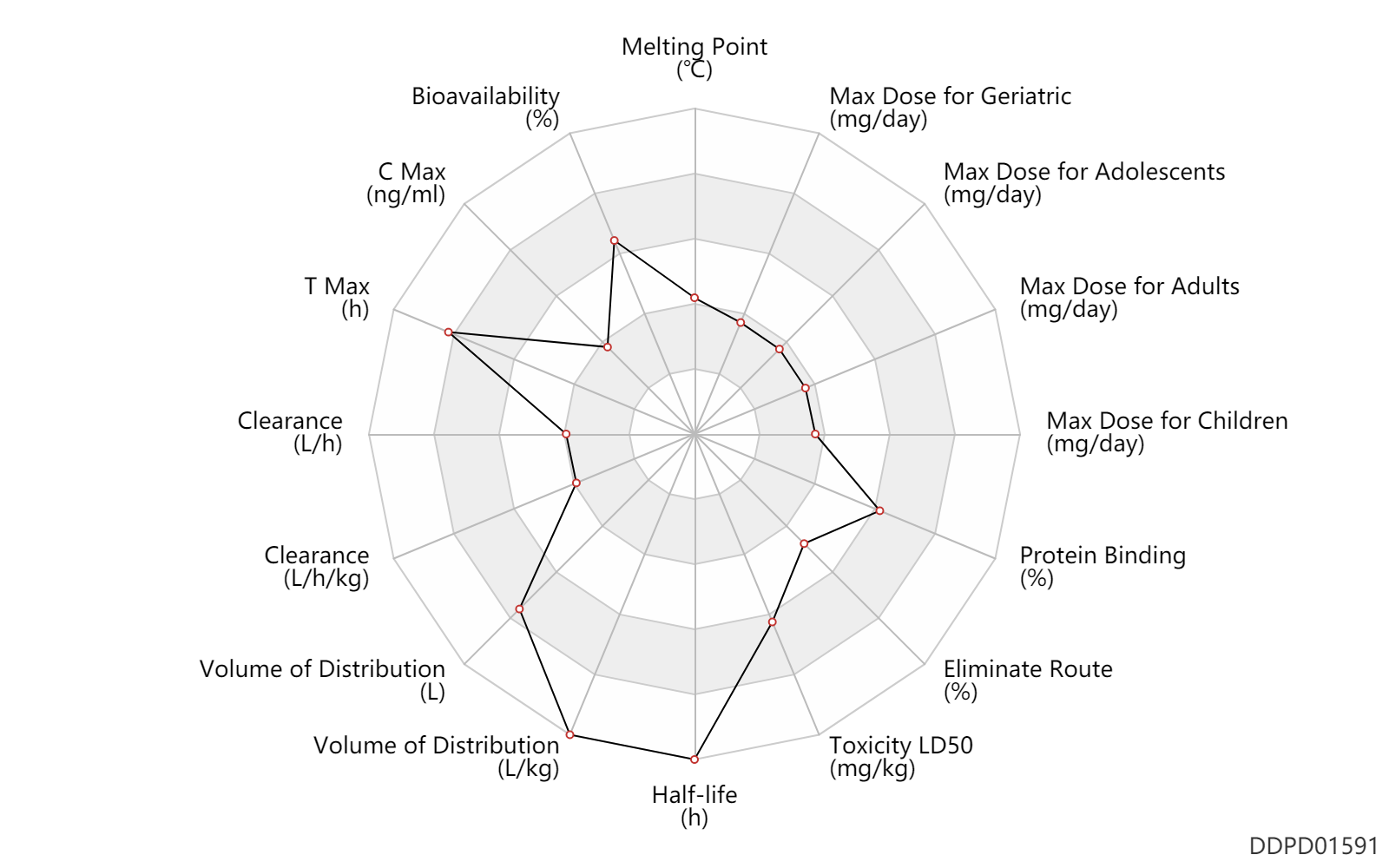
| Melting Point | 135.0 | ℃ | 134-136 | ℃ | https://www.trc-canada.com/product-detail/?S676701 |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
| Bioavailability | 88.0 | % | 88 | % | DRUGBANK | |
| C Max | 32.3 | ng/ml | 32.3 | ng/ml | PO, oral; | DRUGBANK | C Max | 62.9 | ng/ml | 62.9 | ng/ml | PO, oral; | DRUGBANK |
| T Max | 5.5 | h | 3-8 | h | PO, oral; | DRUGBANK |
| Clearance | 10.5 | L/h | 7.0-14 | L/h | DRUGBANK | Clearance | 1.1 | L/h | 0.67-1.51 | L/h | Renal clearance; | DRUGBANK | Clearance | 0.13 | L/h/kg | 2.1 | ml/min/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Volume of Distribution | 600.0 | L | 600.0 | L | DRUGBANK | Volume of Distribution | 8.2 | L/kg | 8.2 | L/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Half-life | 59.0 | h | 33-85 | h | elimination half-life; | DRUGBANK | Half-life | 52.0 | h | 52 | h | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Toxicity LD50 | 3000.0 | mg/kg | 3.0 | g/kg | PO, oral; | DRUGBANK | Toxicity LD50 | 16.9 | mg/kg/h | 186-623 | mg/kg/day | mouse; Rattus, Rat; | DRUGBANK |
| Eliminate Route | 69.2 | % | 69.2±7.8 | % | Urinary excretion; | DRUGBANK | Eliminate Route | 22.5 | % | 22.5±3.3 | % | Faeces excretion; | DRUGBANK | Eliminate Route | 0.40 | % | 0.4±7.8 | % | lung excretion; | DRUGBANK |
| Protein Binding | 94.5 | % | 93-96 | % | plasma proteins; | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for children | 10.0 | mg/day | 10 | mg/day | PO, oral | VESIcare | solifenacin succinate | PDR |
| Max dose for children | 8.0 | mg/day | 8 | mg/day | PO, oral | VESIcare | solifenacin succinate | PDR |
| Max dose for children | 6.0 | mg/day | 6 | mg/day | PO, oral | VESIcare | solifenacin succinate | PDR |
| Max dose for children | 5.0 | mg/day | 5 | mg/day | PO, oral | VESIcare | solifenacin succinate | PDR |
| Max dose for children | 4.0 | mg/day | 4 | mg/day | PO, oral | VESIcare | solifenacin succinate | PDR |
| Max dose for adolescents | 10.0 | mg/day | 10 | mg/day | PO, oral | VESIcare | solifenacin succinate | PDR |
| Max dose for adolescents | 8.0 | mg/day | 8 | mg/day | PO, oral | VESIcare | solifenacin succinate | PDR |
| Max dose for adolescents | 6.0 | mg/day | 6 | mg/day | PO, oral | VESIcare | solifenacin succinate | PDR |
| Max dose for adolescents | 5.0 | mg/day | 5 | mg/day | PO, oral | VESIcare | solifenacin succinate | PDR |
| Max dose for adolescents | 4.0 | mg/day | 4 | mg/day | PO, oral | VESIcare | solifenacin succinate | PDR |
| Max dose for adults | 10.0 | mg/day | 10 | mg/day | PO, oral | VESIcare | solifenacin succinate | PDR |
| Max dose for geriatric | 10.0 | mg/day | 10 | mg/day | PO, oral | VESIcare | solifenacin succinate | PDR |