Basic Information
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
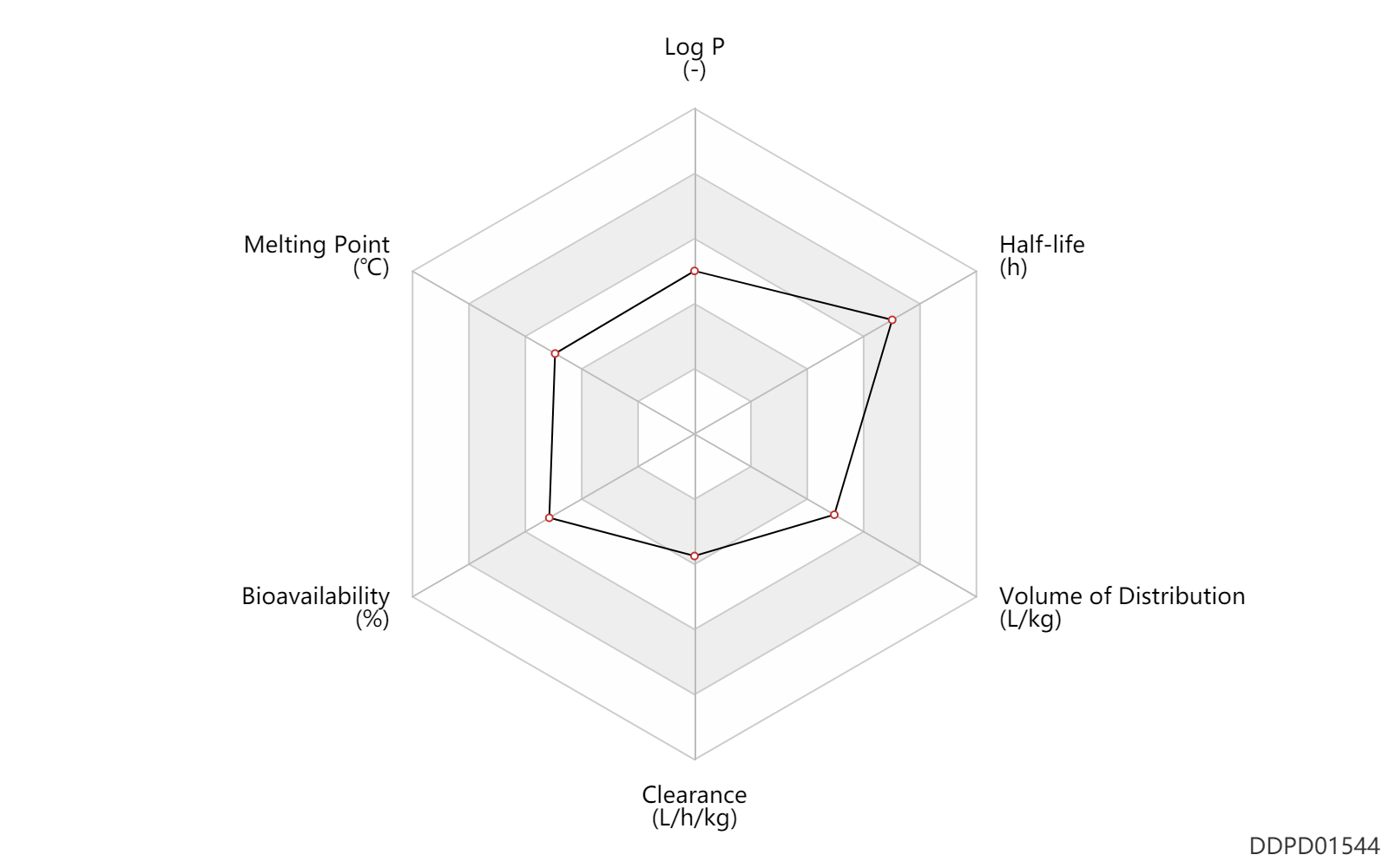
| Log P | 2.06 | - | 2.06 | - | HANSCH,C ET AL. (1995) |
| Melting Point | 166.5 | ℃ | 166-167 | ℃ | Kariss, J. and Newmark, H.L.; U.S. Patent 3,116,203; December 31, 1963; assigned to Hoffmann-la Roche Inc.? Kariss, J. and Newmark, H.L.; US. Patent 3,123,529; March 3,1964; assigned to Hoffmann-La Roche Inc. ?Keiler, O., Steiger, N. and Sternbach, L.H.; U.S. Patent 3,203,990; August 31, 1965; assigned to Hoffmann-La Roche Inc. |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
| Bioavailability | 70.5 | % | 64-77 | % | PO, oral; | DRUGBANK | Bioavailability | 50.0 | % | 50 | % | Rectal Administration; | DRUGBANK |
| Clearance | 0.0840 | L/h/kg | 1.4 | ml/min/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Volume of Distribution | 1.9 | L/kg | 1.9 | L/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Half-life | 22.0 | h | 18-26 | h | DRUGBANK | Half-life | 25.0 | h | 25 | h | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
Maximum Dosage
Not Available