Basic Information
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
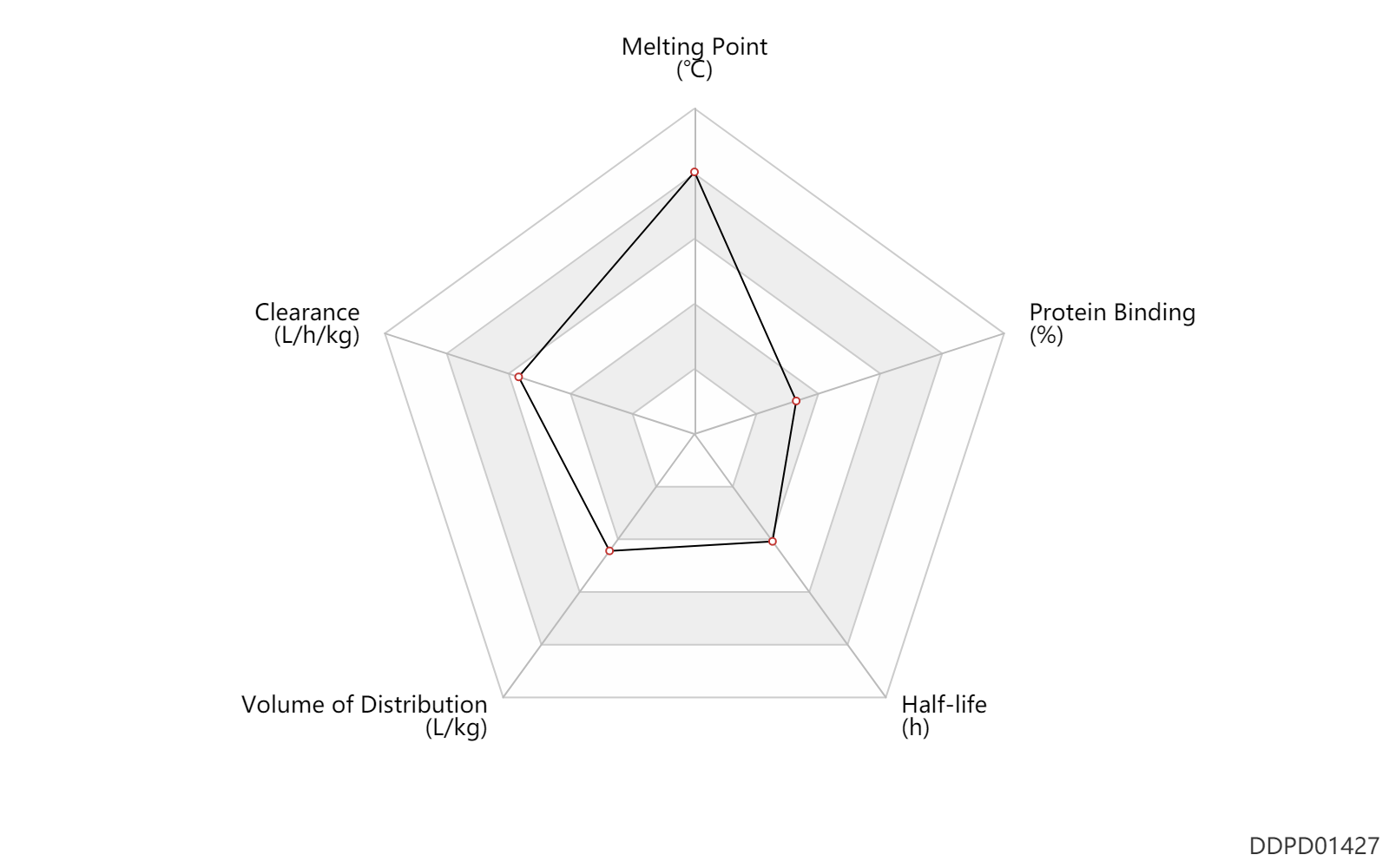
| Melting Point | 295.5 | ℃ | 294-297 | ℃ | Lesher,G.Y. and Opalka, C.J.; US. Patent 4,004,012; January 18,1977; assigned to Sterling Drug Inc. Lesher, G.Y. and Opalka, C.J.; U.S. Patent 4,107,315; August 15,1978; assigned to Sterling Drug Inc. |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
| Clearance | 0.53 | L/h/kg | 8.9 | ml/min/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Volume of Distribution | 1.2 | L/kg | 1.2 | L/kg | normal,healthy; | DRUGBANK | Volume of Distribution | 1.3 | L/kg | 1.3 | L/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Half-life | 6.5 | h | 5-8 | h | DRUGBANK | Half-life | 2.0 | h | 2 | h | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Protein Binding | 29.5 | % | 10-49 | % | DRUGBANK |
Maximum Dosage
Not Available