Basic Information
| Drug ID | DDPD01416 |

|
| Drug Name | Cefpodoxime | |
| Molecular Weight | 427.455 | |
| Molecular Formula | C15H17N5O6S2 | |
| CAS Number | 80210-62-4 | |
| SMILES | [H][C@]12SCC(COC)=C(N1C(=O)[C@H]2NC(=O)C(=N/OC)\C1=CSC(N)=N1)C(O)=O | |
| External Links | ||
| DRUGBANK | DB01416 | |
| PubChem Compound | 6335986 | |
| PDR | 3122 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
Not Available
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
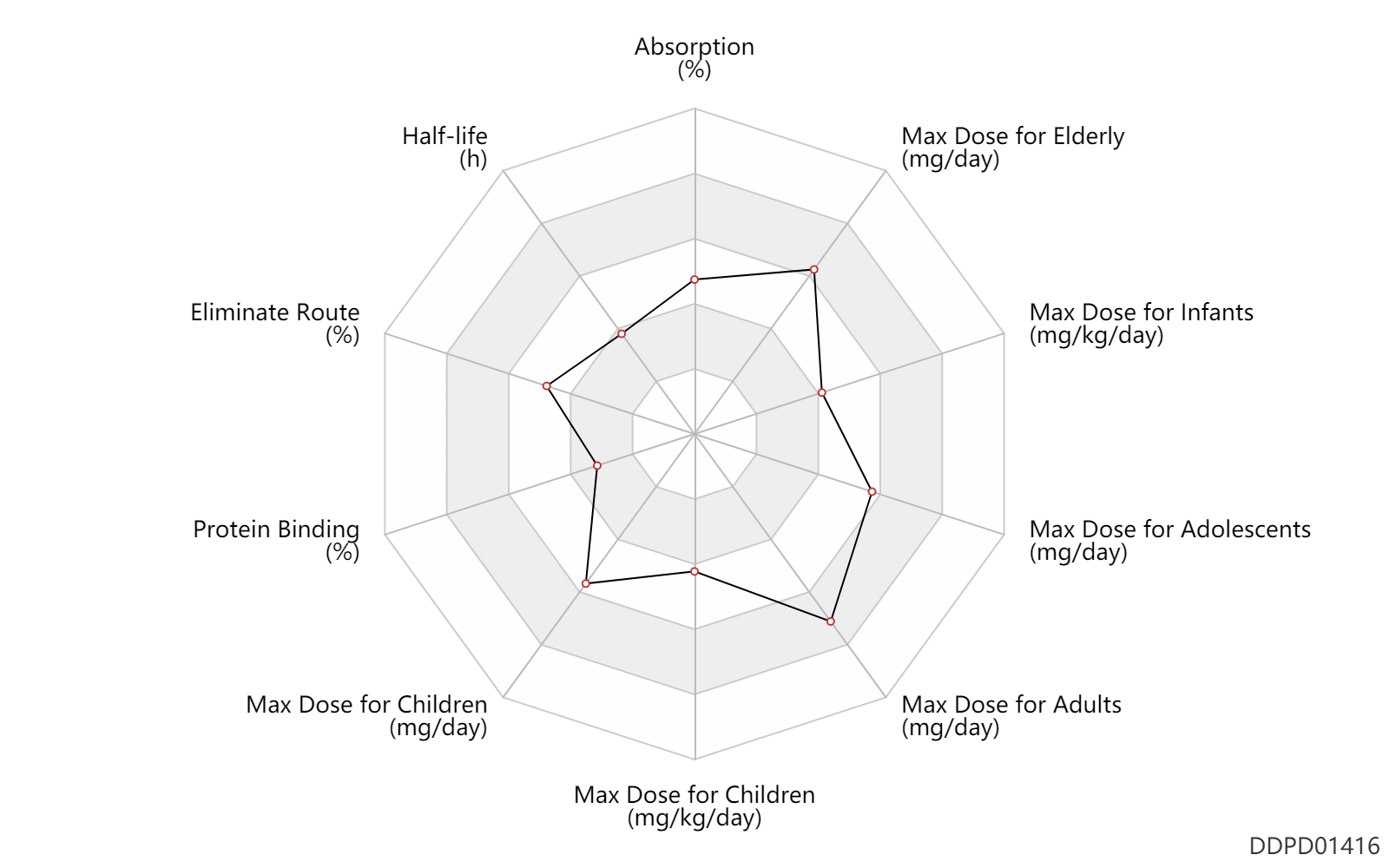
| Absorption | 50.0 | % | 50 | % | PO, oral; fasting; | DRUGBANK |
| Half-life | 2.5 | h | 2.09-2.84 | h | DRUGBANK | |
| Eliminate Route | 31.0 | % | ~29-33 | % | Urinary excretion; Unchanged drug; | DRUGBANK |
| Protein Binding | 27.5 | % | 22-33 | % | DRUGBANK | Protein Binding | 25.0 | % | 21-29 | % | plasma proteins; | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for infants | 10.0 | mg/kg/day | 10 | mg/kg/day | PO, oral | Cefpodoxime Proxetil Oral Suspension | cefpodoxime proxetil | PDR |
| Max dose for children | 800.0 | mg/day | 800 | mg/day | PO, oral | Cefpodoxime Proxetil Oral Suspension | cefpodoxime proxetil | PDR |
| Max dose for children | 10.0 | mg/kg/day | 10 | mg/kg/day | PO, oral | Cefpodoxime Proxetil Oral Suspension | cefpodoxime proxetil | PDR |
| Max dose for children | 400.0 | mg/day | 400 | mg/day | PO, oral | Cefpodoxime Proxetil Oral Suspension | cefpodoxime proxetil | PDR |
| Max dose for children | 200.0 | mg/day | 200 | mg/day | PO, oral | Cefpodoxime Proxetil Oral Suspension | cefpodoxime proxetil | PDR |
| Max dose for adolescents | 800.0 | mg/day | 800 | mg/day | PO, oral | Cefpodoxime Proxetil Oral Suspension | cefpodoxime proxetil | PDR |
| Max dose for adults | 800.0 | mg/day | 800 | mg/day | PO, oral | Cefpodoxime Proxetil Oral Suspension | cefpodoxime proxetil | PDR |
| Max dose for elderly | 800.0 | mg/day | 800 | mg/day | PO, oral | Cefpodoxime Proxetil Oral Suspension | cefpodoxime proxetil | PDR |