Basic Information
| Drug ID | DDPD01410 |

|
| Drug Name | Ciclesonide | |
| Molecular Weight | 540.697 | |
| Molecular Formula | C32H44O7 | |
| CAS Number | 126544-47-6 | |
| SMILES | [H][C@@]12C[C@@]3([H])[C@]4([H])CCC5=CC(=O)C=C[C@]5(C)[C@@]4([H])[C@@H](O)C[C@]3(C)[C@@]1(O[C@@H](O2)C1CCCCC1)C(=O)COC(=O)C(C)C | |
| External Links | ||
| DRUGBANK | DB01410 | |
| PubChem Compound | 6918155 | |
| PDR | 1248 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
Not Available
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
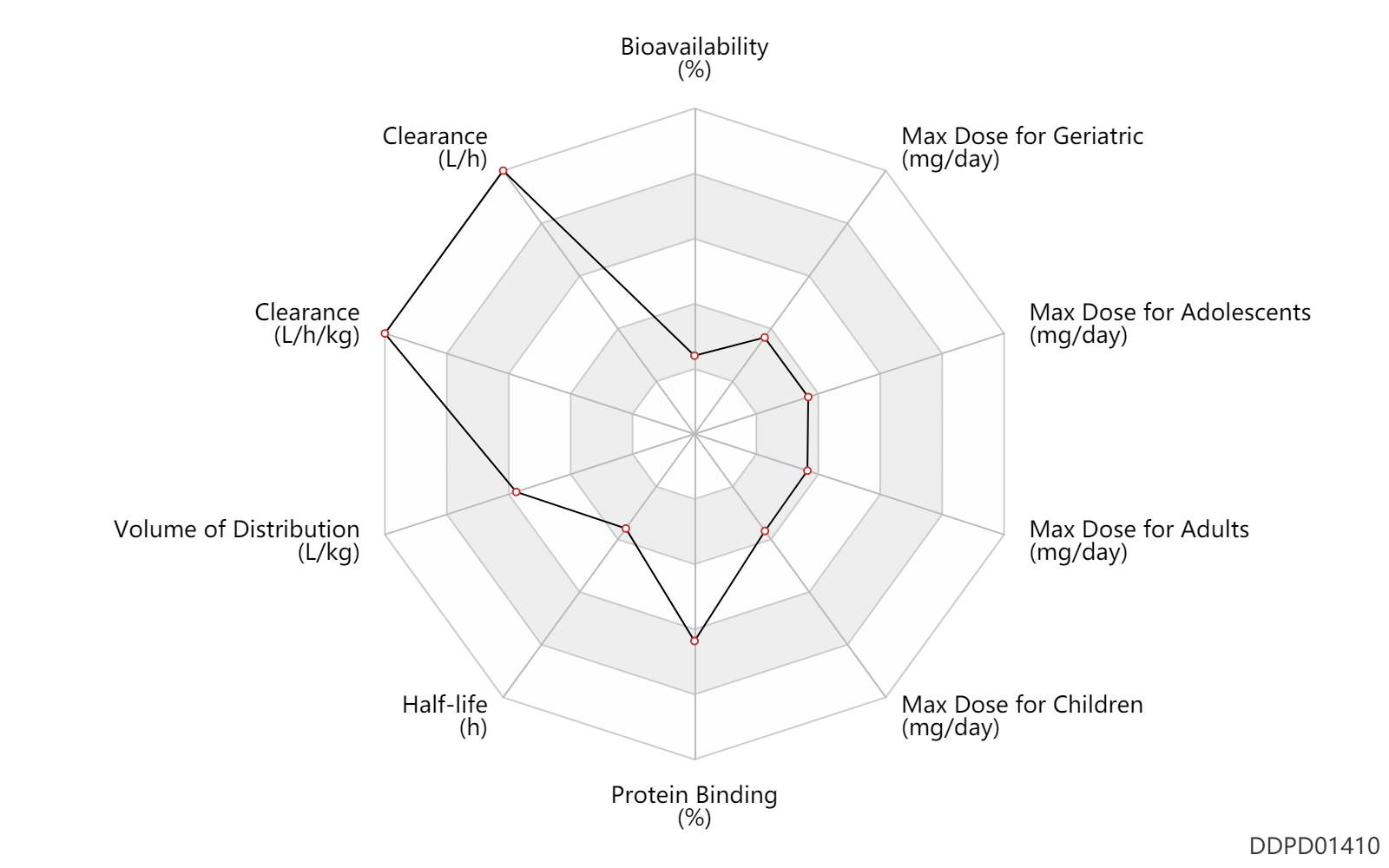
| Bioavailability | 1.0 | % | <1 | % | PO, oral; | DRUGBANK |
| Clearance | 152.0 | L/h | 152.0 | L/h | intravenous injection, IV; | DRUGBANK | Clearance | 2.0 | L/h/kg | 33.3 | ml/min/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Volume of Distribution | 2.9 | L/kg | 2.9 | L/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Half-life | 1.0 | h | 1.04 | h | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Protein Binding | 99.0 | % | 99 | % | plasma proteins; human, homo sapiens; | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Frequency | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Max dose for children | 0.2 | mg/day | 200 | mcg/day | intranasal | qd | Omnaris | ciclesonide | PDR |
| Max dose for children | 0.074 | mg/day | 74 | mcg/day | intranasal | qd | Omnaris | ciclesonide | PDR |
| Max dose for children | 0.64 | mg/day | 640 | mcg/day | inhalation, IH | Omnaris | ciclesonide | PDR | |
| Max dose for children | 0.2 | mg/day | 200 | mcg/day | intranasal | Omnaris | ciclesonide | PDR | |
| Max dose for children | 0.16 | mg/day | 160 | mcg/day | inhalation, IH | Omnaris | ciclesonide | PDR | |
| Max dose for children | 0.16 | mg/day | 160 | mcg/day | inhalation, IH | Omnaris | ciclesonide | PDR | |
| Max dose for adolescents | 0.2 | mg/day | 200 | mcg/day | intranasal | qd | Omnaris | ciclesonide | PDR |
| Max dose for adolescents | 0.074 | mg/day | 74 | mcg/day | intranasal | qd | Omnaris | ciclesonide | PDR |
| Max dose for adolescents | 0.64 | mg/day | 640 | mcg/day | inhalation, IH | Omnaris | ciclesonide | PDR | |
| Max dose for adults | 0.2 | mg/day | 200 | mcg/day | intranasal | Omnaris | ciclesonide | PDR | |
| Max dose for adults | 0.074 | mg/day | 74 | mcg/day | intranasal | Omnaris | ciclesonide | PDR | |
| Max dose for adults | 0.64 | mg/day | 640 | mcg/day | inhalation, IH | Omnaris | ciclesonide | PDR | |
| Max dose for geriatric | 0.2 | mg/day | 200 | mcg/day | intranasal | Omnaris | ciclesonide | PDR | |
| Max dose for geriatric | 0.074 | mg/day | 74 | mcg/day | intranasal | Omnaris | ciclesonide | PDR | |
| Max dose for geriatric | 0.64 | mg/day | 640 | mcg/day | inhalation, IH | Omnaris | ciclesonide | PDR |