Basic Information
| Drug ID | DDPD01406 |

|
| Drug Name | Danazol | |
| Molecular Weight | 337.4553 | |
| Molecular Formula | C22H27NO2 | |
| CAS Number | 17230-88-5 | |
| SMILES | [H][C@@]12CC[C@@](O)(C#C)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CCC2=CC3=C(C[C@]12C)C=NO3 | |
| External Links | ||
| DRUGBANK | DB01406 | |
| PubChem Compound | 28417 | |
| PDR | 1265 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
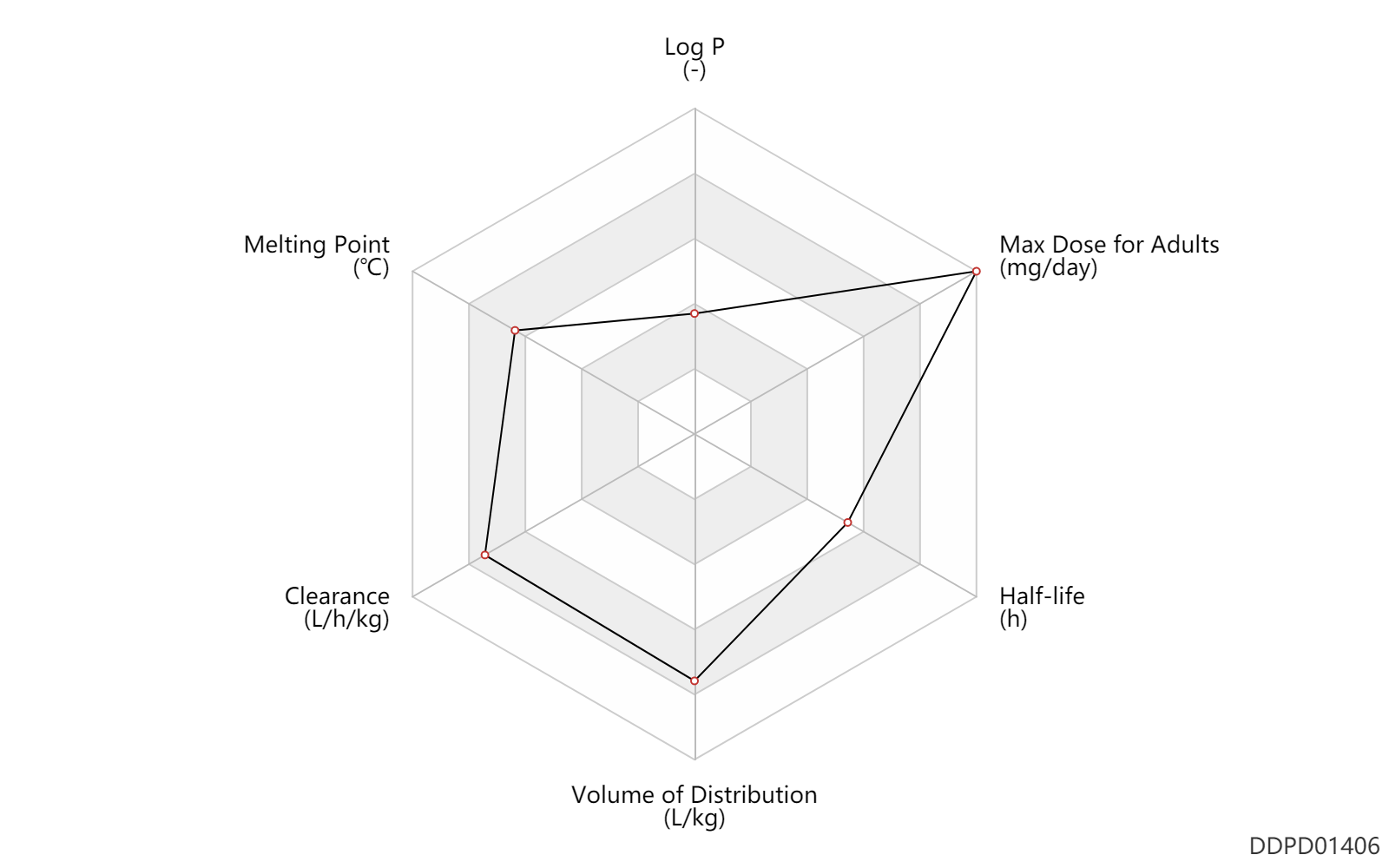
| Log P | 0.51 | - | 0.51 | - | PERRISSOUD,D & TESTA,B (1986) |
| Melting Point | 225.5 | ℃ | 224.2-226.8 | ℃ | Clinton, R. and Hanson, A.; US. Patent 3,135,743; June 2, 1964; assigned to Sterling Drug. |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
| Clearance | 0.94 | L/h/kg | 15.71 | ml/min/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Volume of Distribution | 5.2 | L/kg | 5.18 | L/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Half-life | 24.0 | h | ~24 | h | DRUGBANK | Half-life | 2.3 | h | 2.31 | h | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for adults | 800.0 | mg/day | 800 | mg/day | PO, oral | Danazol | danazol | PDR |
| Max dose for adults | 2400.0 | mg/day | 2400 | mg/day | PO, oral | Danazol | danazol | PDR |