Basic Information
| Drug ID | DDPD01394 |

|
| Drug Name | Colchicine | |
| Molecular Weight | 399.437 | |
| Molecular Formula | C22H25NO6 | |
| CAS Number | 64-86-8 | |
| SMILES | COC1=CC2=C(C(OC)=C1OC)C1=CC=C(OC)C(=O)C=C1C(CC2)NC(C)=O | |
| External Links | ||
| DRUGBANK | DB01394 | |
| T3DB | T3D4020 | |
| PubChem Compound | 2833 | |
| PDR | 3805 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
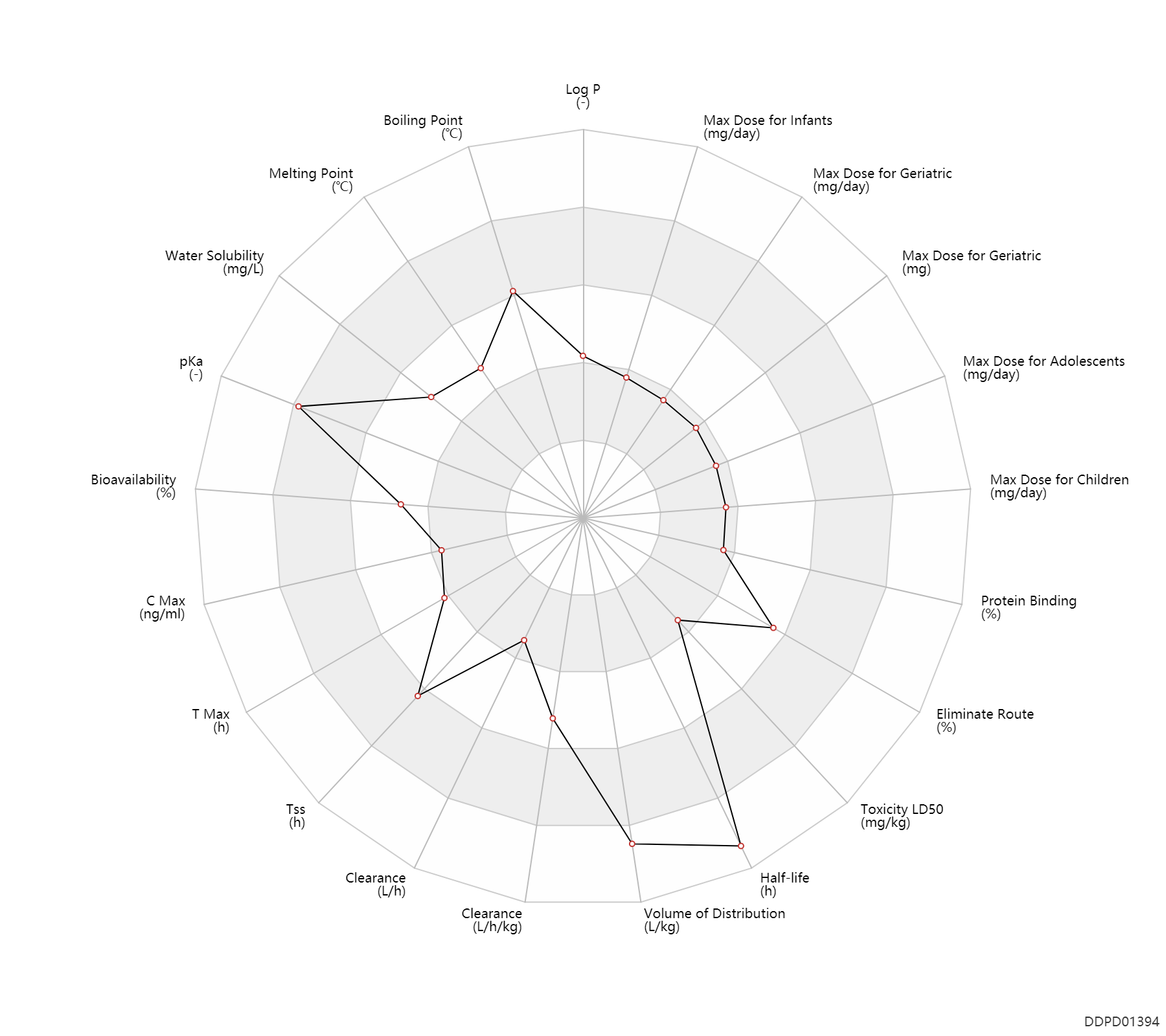
| Log P | 1.07 | - | 1.07 | - | https://www.ebi.ac.uk/chembl/compound_report_card/CHEMBL107/ |
| Boiling Point | 522.37 | ℃ | 522.37 | ℃ | https://www.chemicalbook.com/ChemicalProductProperty_US_CB6391144.aspx |
| Melting Point | 155.0 | ℃ | 150-160 | ℃ | https://www.chemicalbook.com/ChemicalProductProperty_US_CB6391144.aspx |
| Water Solubility | 10000.0 | mg/L | 10 | mg/ml | https://www.chemicalbook.com/ChemicalProductProperty_US_CB6391144.aspx |
| pKa | 12.36 | - | 12.36 | - | https://www.chemicalbook.com/ChemicalProductProperty_US_CB6391144.aspx |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Factor | Reference |
|---|---|---|---|---|---|---|---|
| Bioavailability | 45.0 | % | 45 | % | PO, oral; | DRUGBANK | Bioavailability | 56.0 | % | 24-88 | % | PO, oral; different study; | DRUGBANK |
| C Max | 2.5 | ng/ml | 2.5 | ng/ml | PO, oral; | DRUGBANK | |
| T Max | 1.5 | h | 1-2 | h | PO, oral; | DRUGBANK | |
| Tss | 192.0 | h | 8 | day | Oral multiple dose; | DRUGBANK | |
| Clearance | 0.001839 | L/h | 0.0292-0.0321 | ml/min | Oral single dose; | RD, renal impairment, Renal disease,including uremia ↓ ; | DRUGBANK | Clearance | 0.73 | L/h/kg | 0.726±0.110 | L/h/kg | apparent clearance; Familial Mediterranean Fever; patients; | DRUGBANK | Clearance | 0.13 | L/h/kg | 2.1 | ml/min/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Volume of Distribution | 6.5 | L/kg | 5.0-8 | L/kg | Apparent volume of distribution; normal,healthy; patients; young; | DRUGBANK | Volume of Distribution | 6.1 | L/kg | 6.1 | L/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Half-life | 28.9 | h | 26.6-31.2 | h | elimination half-life; | DRUGBANK | Half-life | 30.0 | h | 20-40 | h | different study; | DRUGBANK | Half-life | 58.0 | h | 58 | h | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Toxicity LD50 | 5.9 | mg/kg | 5.87 | mg/kg | PO, oral; mouse; | DRUGBANK | |
| Eliminate Route | 52.5 | % | 40-65 | % | Urinary excretion; normal,healthy; human, homo sapiens; Unchanged drug; | DRUGBANK | |
| Protein Binding | 39.0 | % | 39±5 | % | plasma proteins; | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for infants | 1.8 | mg/day | 1.8 | mg/day | Tablet,PO,oral | Mitigare | colchicine | PDR |
| Max dose for children | 2.4 | mg/day | 2.4 | mg/day | Tablet,PO,oral | Mitigare | colchicine | PDR |
| Max dose for children | 1.2 | mg/day | 1.2 | mg/day | PO, oral; rarely | Mitigare | colchicine | PDR |
| Max dose for adolescents | 1.8 | mg | 1.8 | mg | Tablet,PO,oral | Mitigare | colchicine | PDR |
| Max dose for adolescents | 1.2 | mg/day | 1.2 | mg/day | Tablet,PO,oral;Capsule, PO, Oral;Liquid; | Mitigare | colchicine | PDR |
| Max dose for adolescents | 2.4 | mg/day | 2.4 | mg/day | Tablet,PO,oral | Mitigare | colchicine | PDR |
| Max dose for geriatric | 1.8 | mg | 1.8 | mg | Tablet,PO,oral | Mitigare | colchicine | PDR |
| Max dose for geriatric | 1.2 | mg/day | 1.2 | mg/day | Tablet,PO,oral;Capsule, PO, Oral;Liquid; | Mitigare | colchicine | PDR |
| Max dose for geriatric | 2.4 | mg/day | 2.4 | mg/day | Tablet,PO,oral | Mitigare | colchicine | PDR |