Basic Information
| Drug ID | DDPD01364 |

|
| Drug Name | Ephedrine | |
| Molecular Weight | 165.2322 | |
| Molecular Formula | C10H15NO | |
| CAS Number | 299-42-3 | |
| SMILES | CN[C@@H](C)[C@H](O)C1=CC=CC=C1 | |
| External Links | ||
| DRUGBANK | DB01364 | |
| T3DB | T3D3040 | |
| PubChem Compound | 9294 | |
| PDR | 23927 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
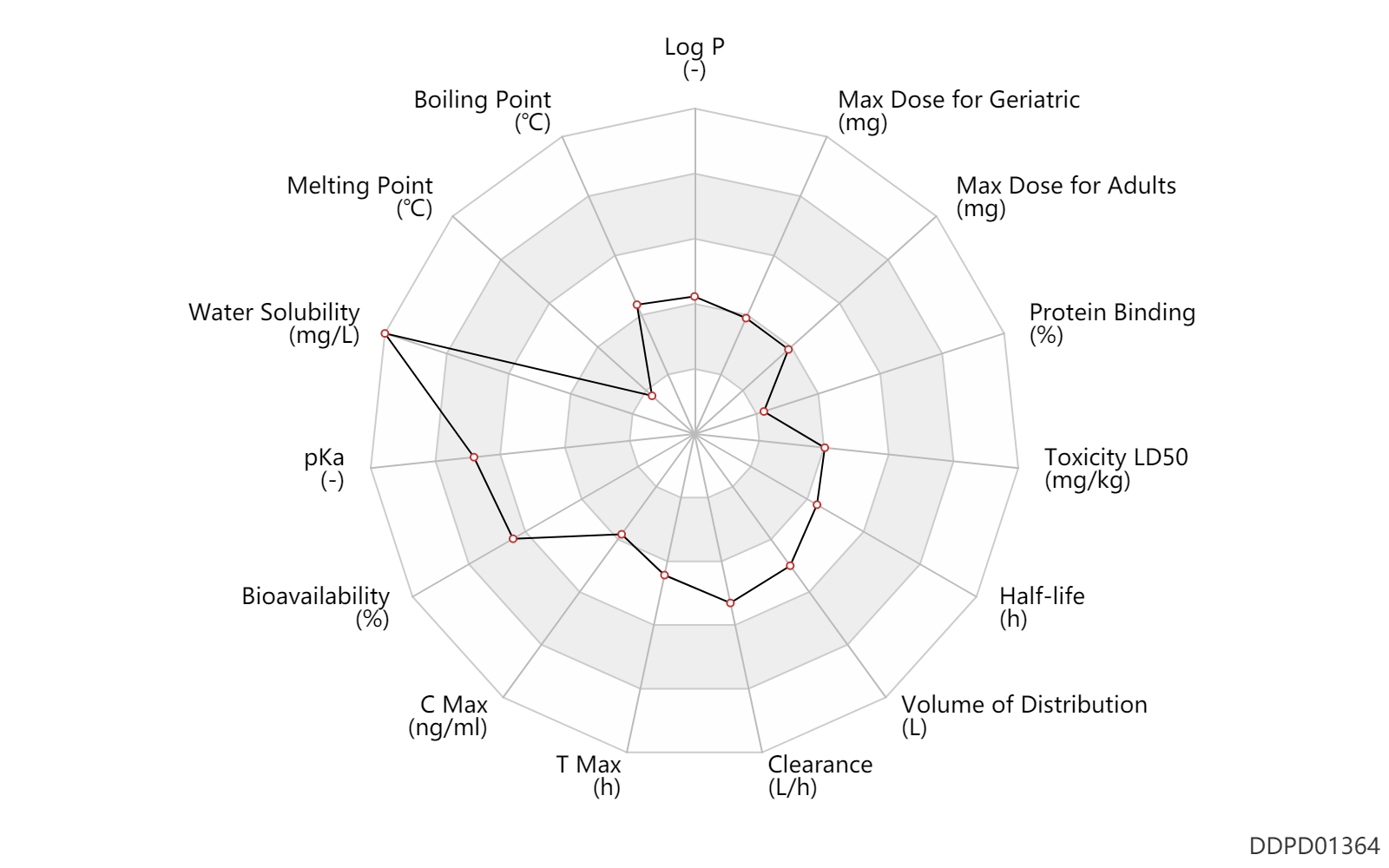
| Log P | 1.13 | - | 1.13 | - | AVDEEF,A (1997) |
| Boiling Point | 255.0 | ℃ | 255 | ℃ | PhysProp |
| Melting Point | 34.0 | ℃ | 34 | ℃ | PhysProp |
| Water Solubility | 63600.0 | mg/L | 63600 | mg/L | YALKOWSKY,SH & DANNENFELSER,RM 1992) |
| pKa | 10.3 | - | 10.3 | - | PERRIN,DD (1965) |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
| Bioavailability | 88.0 | % | 88 | % | PO, oral; | DRUGBANK |
| C Max | 79.5 | ng/ml | 79.5 | ng/ml | PO, oral; | DRUGBANK |
| T Max | 1.8 | h | 1.81 | h | PO, oral; | DRUGBANK |
| Clearance | 23.3 | L/h | 23.3 | L/h | PO, oral; | DRUGBANK |
| Volume of Distribution | 215.6 | L | 215.6 | L | Average volume of distribution; PO, oral; | DRUGBANK |
| Half-life | 6.0 | h | ~6 | h | elimination half-life; PO, oral; | DRUGBANK |
| Toxicity LD50 | 785.0 | mg/kg | 785.0 | mg/kg | PO, oral; mouse; | DRUGBANK | Toxicity LD50 | 284.0 | mg/kg | 284.0 | mg/kg | Intraperitoneal, IP; mouse; | DRUGBANK | Toxicity LD50 | 425.0 | mg/kg | 425.0 | mg/kg | subcutaneous injection, SC; mouse; | DRUGBANK |
| Protein Binding | 4.9 | % | 4.9±0.3 | % | Raceme L; human, homo sapiens; | DRUGBANK | Protein Binding | 6.9 | % | 6.9±1.4 | % | Raceme D; human, homo sapiens; | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for adults | 50.0 | mg | 50 | mg | intravenous injection, IV | Akovaz | ephedrine sulfate | PDR |
| Max dose for geriatric | 50.0 | mg | 50 | mg | intravenous injection, IV | Akovaz | ephedrine sulfate | PDR |