Basic Information
| Drug ID | DDPD01332 |

|
| Drug Name | Ceftizoxime | |
| Molecular Weight | 383.403 | |
| Molecular Formula | C13H13N5O5S2 | |
| CAS Number | 68401-81-0 | |
| SMILES | [H][C@]12SCC=C(N1C(=O)[C@H]2NC(=O)C(=N/OC)\C1=CSC(N)=N1)C(O)=O | |
| External Links | ||
| DRUGBANK | DB01332 | |
| PubChem Compound | 6533629 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
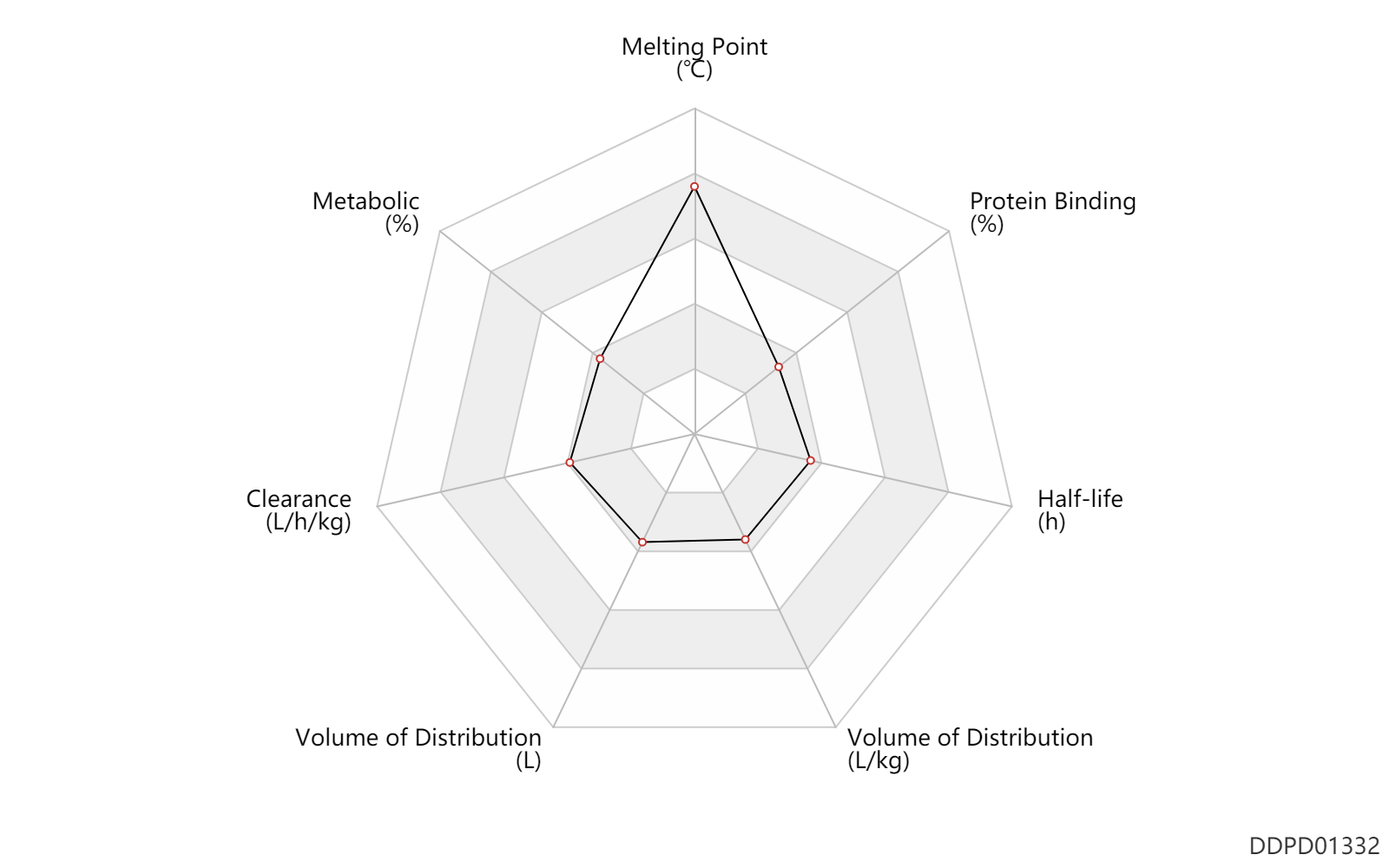
| Melting Point | 277.0 | ℃ | 277 | ℃ | Takaya, T., Masugi, T., Takasugi, H. and Kochi, H.;U.S. Patent 4,166,115; assigned to Fuji- sawa Pharmaceutical & ., Ltd. |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
| Metabolic | 0 | % | 0 | % | DRUGBANK | Metabolic | 0 | % | ~0 | % | Urinary excretion; | DRUGBANK |
| Clearance | 0.13 | L/h/kg | 2.1 | ml/min/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Volume of Distribution | 21.5 | L | 15-28 | L | Apparent volume of distribution; | DRUGBANK | Volume of Distribution | 0.20 | L/kg | 0.2 | L/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Half-life | 1.5 | h | 1.5 | h | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Protein Binding | 30.0 | % | 30 | % | DRUGBANK |
Maximum Dosage
Not Available