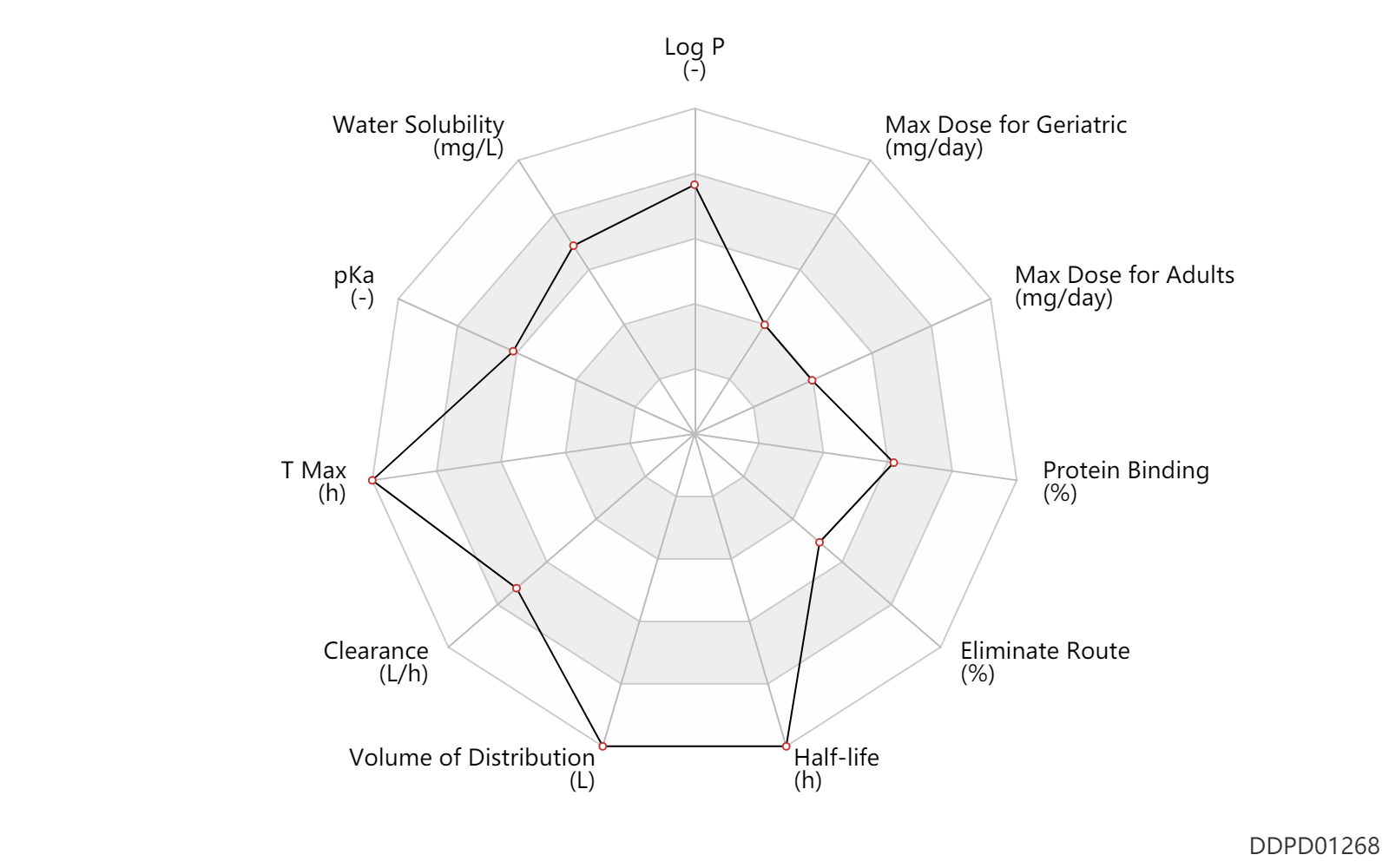
Basic Information
| Drug ID | DDPD01268 |

|
| Drug Name | Sunitinib | |
| Molecular Weight | 398.4738 | |
| Molecular Formula | C22H27FN4O2 | |
| CAS Number | 557795-19-4 | |
| SMILES | CCN(CC)CCNC(=O)C1=C(C)NC(\C=C2/C(=O)NC3=C2C=C(F)C=C3)=C1C | |
| External Links | ||
| DRUGBANK | DB01268 | |
| PubChem Compound | 5329102 | |
| PDR | 470 | |
| Drugs.com | Drugs.com Drug Page | |
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
| Log P | 5.2 | - | 5.2 | - | FDA label |
| Water Solubility | 25000.0 | mg/L | >25 | mg/ml | FDA label |
| pKa | 8.95 | - | 8.95 | - | FDA label |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
| T Max | 9.0 | h | 6-12 | h | PO, oral; | DRUGBANK |
| Clearance | 48.0 | L/h | 34-62 | L/h | Total clearance; PO, oral; | DRUGBANK |
| Volume of Distribution | 2230.0 | L | 2230.0 | L | Apparent volume of distribution; | DRUGBANK |
| Half-life | 50.0 | h | 40-60 | h | terminal half-life; Oral single dose; normal,healthy; | DRUGBANK | Half-life | 95.0 | h | 80-110 | h | terminal half-life; Active metabolite; Oral single dose; normal,healthy; | DRUGBANK |
| Toxicity MTD | 500.0 | mg/kg | >500 | mg/kg | PO, oral; Rattus, Rat; mouse; dog; | DRUGBANK | Toxicity MTD | 1200.0 | mg/kg | >1200 | mg/kg | non-human primate; | DRUGBANK |
| Eliminate Route | 61.0 | % | 61 | % | Faeces excretion; | DRUGBANK | Eliminate Route | 16.0 | % | 16 | % | Urinary excretion; | DRUGBANK |
| Protein Binding | 95.0 | % | 95 | % | plasma proteins; high protein binding; human, homo sapiens; | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for adults | 87.5 | mg/day | 87.5 | mg/day | PO, oral | Sutent | sunitinib malate | PDR |
| Max dose for adults | 62.5 | mg/day | 62.5 | mg/day | PO, oral | Sutent | sunitinib malate | PDR |
| Max dose for geriatric | 87.5 | mg/day | 87.5 | mg/day | PO, oral | Sutent | sunitinib malate | PDR |
| Max dose for geriatric | 62.5 | mg/day | 62.5 | mg/day | PO, oral | Sutent | sunitinib malate | PDR |