Basic Information
| Drug ID | DDPD01264 |

|
| Drug Name | Darunavir | |
| Molecular Weight | 547.664 | |
| Molecular Formula | C27H37N3O7S | |
| CAS Number | 206361-99-1 | |
| SMILES | [H][C@@]12CCO[C@]1([H])OC[C@@H]2OC(=O)N[C@@H](CC1=CC=CC=C1)[C@H](O)CN(CC(C)C)S(=O)(=O)C1=CC=C(N)C=C1 | |
| External Links | ||
| DRUGBANK | DB01264 | |
| PubChem Compound | 213039 | |
| PDR | 1229 | |
| Drugs.com | Drugs.com Drug Page | |
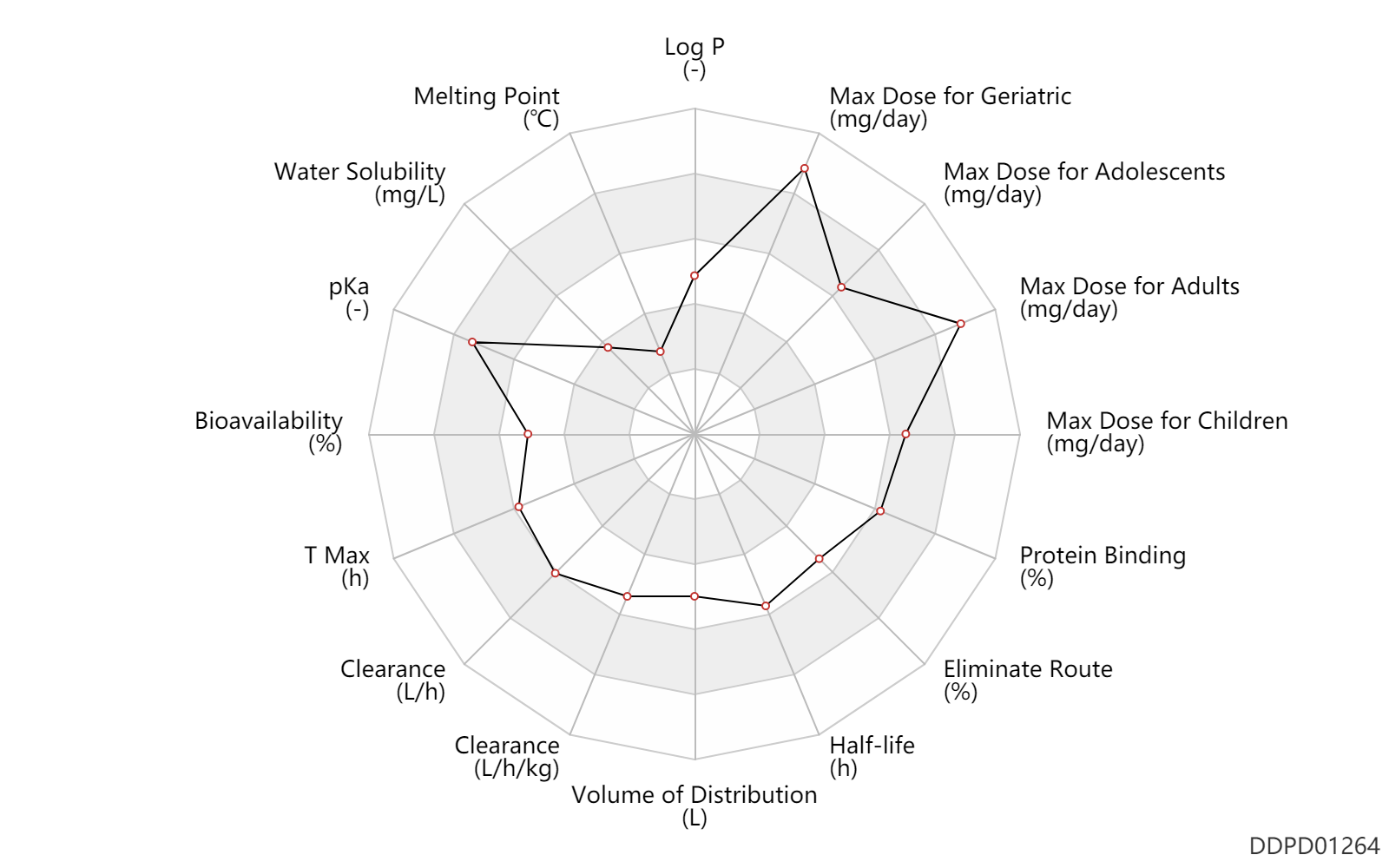
Experimental Physicochemical Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Reference |
|---|---|---|---|---|---|
| Log P | 1.89 | - | 1.89 | - | http://www.antimicrobe.org/d94.asp |
| Melting Point | 75.0 | ℃ | 74-76 | ℃ | https://www.chemicalbook.com/ChemicalProductProperty_EN_CB61121362.htm |
| Water Solubility | 150.0 | mg/L | 0.15 | mg/ml | https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021976s003s004lbl.pdf |
| pKa | 11.43 | - | 11.43±0.46 | - | https://www.chemicalbook.com/ChemicalProductProperty_US_CB51176244.aspx |
Pharmacokinetic/ Toxicokinetic Property
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Reference |
|---|---|---|---|---|---|---|
| Bioavailability | 37.0 | % | 37 | % | Oral single dose; | DRUGBANK | Bioavailability | 82.0 | % | 82 | % | combination drug use; | DRUGBANK |
| T Max | 3.2 | h | 2.4-4 | h | PO, oral; | DRUGBANK |
| Clearance | 32.8 | L/h | 32.8 | L/h | intravenous injection, IV; | DRUGBANK | Clearance | 0.47 | L/h/kg | 7.81 | ml/min/kg | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Volume of Distribution | 206.5 | L | 206.5 | L | Drug combination; | DRUGBANK | Volume of Distribution | 220.0 | L | 220.0 | L | DRUGBANK |
| Half-life | 15.0 | h | ~15 | h | elimination half-life; Drug combination; | DRUGBANK | Half-life | 15.0 | h | 15 | h | intravenous injection, IV; human, homo sapiens; | Human Intravenous Pharmacokinetic Dataset |
| Eliminate Route | 79.5 | % | ~79.5 | % | Faeces excretion; Single dose; Drug combination; normal,healthy; human, homo sapiens; | DRUGBANK | Eliminate Route | 13.9 | % | ~13.9 | % | Urinary excretion; Single dose; Drug combination; Ritonavir; normal,healthy; human, homo sapiens; | DRUGBANK |
| Protein Binding | 95.0 | % | ~95 | % | plasma proteins; | DRUGBANK |
Maximum Dosage
| Property Name | Property Value | Unit | Raw Value | Raw Unit | Annotation | Brand Name | Component | Reference |
|---|---|---|---|---|---|---|---|---|
| Max dose for children | 1200.0 | mg/day | 1200 | mg/day | PO, oral | Prezista | darunavir | PDR |
| Max dose for children | 900.0 | mg/day | 900 | mg/day | PO, oral | Prezista | darunavir | PDR |
| Max dose for children | 750.0 | mg/day | 750 | mg/day | PO, oral | Prezista | darunavir | PDR |
| Max dose for children | 560.0 | mg/day | 560 | mg/day | PO, oral | Prezista | darunavir | PDR |
| Max dose for children | 520.0 | mg/day | 520 | mg/day | PO, oral | Prezista | darunavir | PDR |
| Max dose for children | 480.0 | mg/day | 480 | mg/day | PO, oral | Prezista | darunavir | PDR |
| Max dose for children | 440.0 | mg/day | 440 | mg/day | PO, oral | Prezista | darunavir | PDR |
| Max dose for children | 400.0 | mg/day | 400 | mg/day | PO, oral | Prezista | darunavir | PDR |
| Max dose for adolescents | 1200.0 | mg/day | 1200 | mg/day | PO, oral | Prezista | darunavir | PDR |
| Max dose for adolescents | 900.0 | mg/day | 900 | mg/day | PO, oral | Prezista | darunavir | PDR |
| Max dose for adults | 1200.0 | mg/day | 1200 | mg/day | PO, oral | Prezista | darunavir | PDR |
| Max dose for geriatric | 1200.0 | mg/day | 1200 | mg/day | PO, oral | Prezista | darunavir | PDR |